Neuroscience


This theme’s focus:
- To develop personalised treatments for patients with brain diseases to reduce disability and prolong a healthy life
- We focus on rare inherited brain and muscle disorders, brain inflammation including multiple sclerosis, and traumatic brain injury
Neurological disorders affect one in six in the UK and are complex and highly variable, with limited treatment opportunities.
Stroke and dementia account for less than 15% of neurological disorders. Rare neurological disorders affect around one in 1,000, conditions of brain inflammation, including multiple sclerosis (MS) and autoimmune encephalitis (AE) affect around four in 1,000; whereas traumatic brain injury (TBI) is the leading cause of death in young adults and a major cause of disability affecting 50 million people every year worldwide.
NHS hospitals have seen more than a 20% increase in neurological admissions since 2016, which reflects advances in the management of acute neurological trauma and an ageing population. This leaves many patients with life-long disability, has substantial negative impact on quality of life, and costs the NHS over £4.4billion a year.
To address this, we want to use technological advances in genomics, metabolomics (the study of small molecules in the body, known as metabolites) and imaging to help us develop personalised medicine in rare neurological disorders, brain inflammation and traumatic brain injury.
We have established a region-wide hub for precision experimental medicine and early phase interventions in neurological diseases, recruiting participants across the East of England and nationally, reaching deep into deprived rural and coastal areas.
And our dedicated Patient and Public Involvement (PPI) panel helps us by providing detailed input into grant applications and shaping our research priorities. We have hosted visits from patients, friends and relatives, alongside traditional PPI outreach events. All of our clinical study groups include one or more PPI representatives.
Here are some of the conditions we are researching:
Brain inflammation
Brain inflammation includes multiple sclerosis (MS) and encephalitis.
MS has many treatments but we don’t know much about their effectiveness outside short clinical trials, and there are no treatments that promote remyelination, which can prevent long-term neurodegeneration in MS.
Encephalitis can be viral or autoimmune. Treatments of these are different but diagnostic confirmation takes time, especially for autoimmune encephalitis (AE).
We aim to optimise treatments for multiple sclerosis (MS) and optimise how we detect and stratify (group) both viral and autoimmune encephalitis.

Rare inherited neurological diseases
A genetic diagnosis is not possible for around 40% of families with suspected neurogenetic disorders, even after whole genome sequencing. By providing a molecular diagnosis, we can then enable accurate genetic counselling, prenatal diagnosis and the diagnosis of unrecognised treatable disorders.
We also want to develop new treatments for inherited neurological disorders. The majority of neurogenetic disorders have no treatment, causing progressive disability and early death. Capitalising on our international leadership in mitochondrial biology, we will focus on mitochondrial disorders which affect one in 5,000 people.
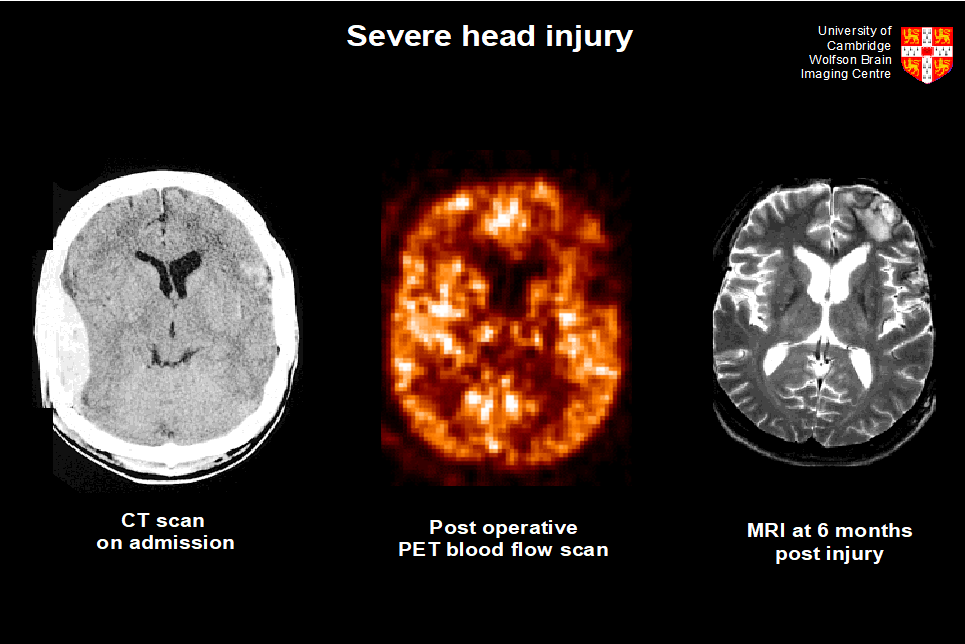
Traumatic brain injury (TBI)
Traumatic brain injury is responsible for many of the 11,000 trauma deaths per day globally. We have developed innovative therapies and identified novel monitoring tools to target the management of intracranial pressure, breaking the vicious cycle of brain swelling. Our work has contributed to reducing the likelihood of dying from brain injury. Specifically, decompressive craniectomy is now used routinely in the UK and has saved 22 additional lives out of 100, with 60% of survivors independent at home.
However, current treatment paradigms are generic and provide no framework for developing and using personalised medicine approaches (particularly in patients with mild injury). They also take no account of the growing evidence that TBI can trigger neurodegeneration. We aim to develop personalised medicine in TBI, and develop and evaluate novel experimental therapies for specific patients groups, to further reduce mortality and disability in survivors.

