COVID-19 case studies
Read about some of the COVID-19 studies the NIHR Cambridge BRC supported.
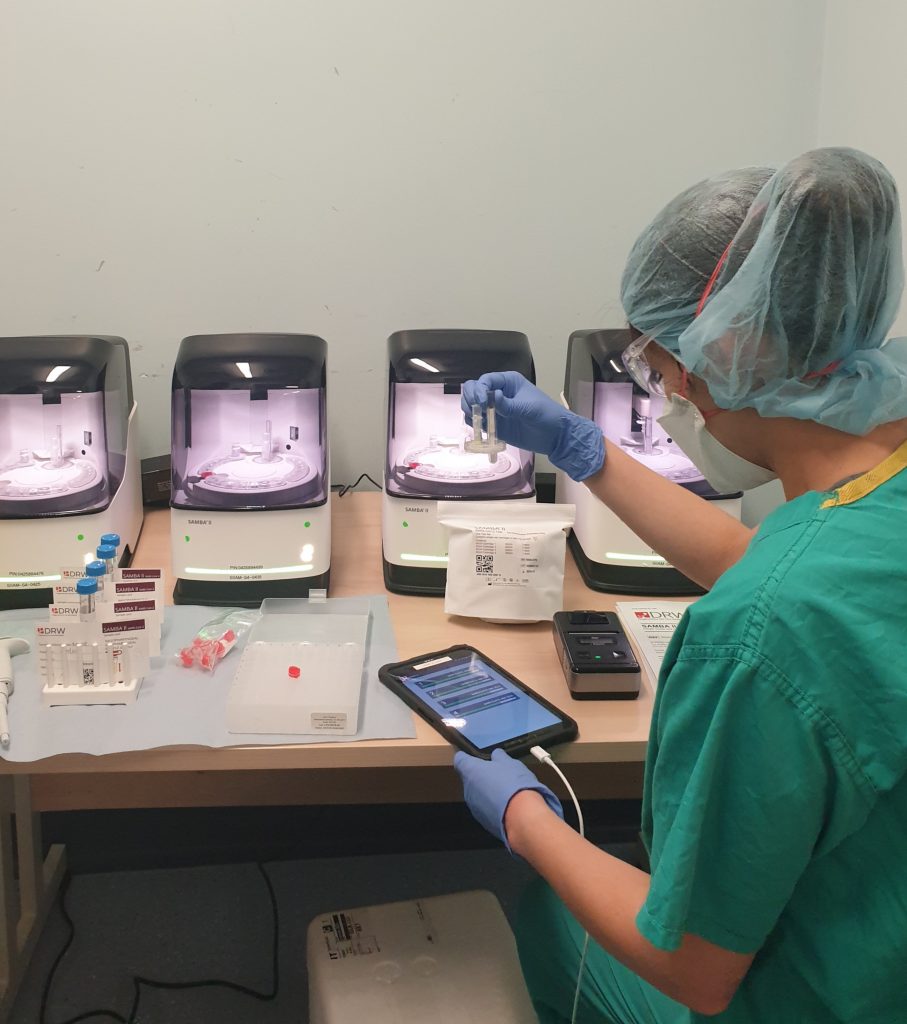
Speeding up the diagnosis of COVID-19 in a hospital setting using a SAMBA II
COVIDx, a study supported by the NIHR Cambridge BRC and NIHR Cambridge CRF, aims to investigate the impact of two new tests for COVID-19 on delivering faster diagnoses and understanding the development of immunity following infection.
The first part of the study will evaluate the accuracy of the new SAMBA II-based test and whether it speeds up the diagnosis of COVID-19 in a ‘real-time’ hospital setting at the point of care.
The second part will investigate a point of care finger prick ‘antibody’ test of the blood, to determine how quickly markers of immunity appear following infection and a positive SAMBA test. These antibody tests will be important for understanding which patients and staff have already had the infection, and may be safe to return to work following recovery.
Research nurses from the NIHR Cambridge CRF are collecting samples from patients with suspected COVID-19 to support the COVIDx study, using the SAMBA II machine to test nasal and throat swabs to determine if a patient has COVID-19 and if the new device is an improved source of testing.
The SAMBA II test can provide extremely reliable results in less than two hours, meaning decisions about clinical care or self-isolation can be made much more rapidly. The antibody tests require serum from blood samples, which will be tested in specialised facilities at the Cambridge Institute for Therapeutic Immunology and Infectious Diseases (CITIID).
Once the two diagnostic tests have been validated in patients with confirmed COVID-19, the study will enrol a second group of participants – healthcare workers. The SAMBA II test will be able to quickly identify staff who are positive for COVID-19, even if they have no symptoms, allowing them to self-isolate or access treatment if required.
This is an abridged version of the news item posted on our website on April 17, 2020.

Cambridge trial targets immune response to treat COVID-19 patients
A new national study, supported by the NIHR Cambridge Biomedical Research Centre and the Cambridge Clinical Trials Unit, will test whether two drugs that are already in use to treat other immune-related conditions can prevent the development of severe COVID-19 infection.
The TACTIC-R trial will target patients as they are admitted to hospital, and test whether drugs that suppress the immune system can prevent the body from ‘over-reacting’ to infection and destroy healthy tissues as well as virus-infected ones, leading to severe COVID-19 disease.
For the majority of people who have COVID-19, the infection causes only mild symptoms including a fever and cough. However, around 15% of patients develop severe disease, which includes serious damage to the lungs and multiple organ failure. This lung and organ damage appears to be mostly caused by the body’s own immune system responding to the presence of infected cells. Researchers hope that preventing the immune ‘over-reaction’ using drugs that stop or ‘suppress’ the immune response will stop patients developing the severest form of COVID-19, preventing the need for intensive care.
TACTIC will initially test two drugs – Ravulizumab and Baricitinib – that used to treat other conditions caused by an overactive immune system.
Ravulizumab is usually used to treat autoimmune conditions where the body destroys red blood cells.
Baricitinib is used to treat people with rheumatoid arthritis.
Both these drugs have been carefully selected by a consortium of doctors and scientists with expertise in treating immune-mediated diseases, and are thought to have a high chance of reducing the immune ‘over-reactions’ seen in very sick patients with COVID-19.
This is an abridged version of the news story that was first published on our website on 16 May 2020.
Single dose of Pfizer BioNTech vaccine reduces asymptomatic infections and potential for SARS-CoV-2 transmission
New data from Addenbrooke’s Hospital suggests that a single dose of the Pfizer BioNTech vaccine can reduce by 75% the number of asymptomatic SARS-CoV-2 infections.
This implies that the vaccine could significantly reduce the risk of transmission of the virus from people who are asymptomatic, as well as protecting others from getting ill.
The study analysed results from thousands of COVID-19 tests carried out each week as part its screening programmes on hospital staff who showed no signs of infection.
The results were then separated out to identify unvaccinated staff, and staff who had been vaccinated more than 12 days prior to testing (when protection against symptomatic infection is thought to occur). The study found that 0·8% of tests from unvaccinated healthcare workers were positive, compared with 0.37% of tests from healthcare workers less than 12 days post-vaccination and 0·2% from healthcare workers at 12 days or more post-vaccination.
This suggests a four-fold decrease in the risk of asymptomatic COVID-19 infection amongst healthcare workers who have been vaccinated for more than 12 days (75 percent protection). The level of asymptomatic infection was also halved in those vaccinated for less than 12 days.
When the team included symptomatic healthcare workers, their analyses showed similar reductions. 1·71% unvaccinated healthcare workers tested positive, compared with 0·4% healthcare workers at 12 or more days post-vaccination.
This is an abridged version of the press release which was first published on our website on March 2, 2021.
Pfizer BioNTech vaccine likely to be effective against B1.1.7 strain of SARS-CoV-2
The Pfizer BioNTech vaccine BNT162b2 is likely to be effective against the B1.1.7 variant of SARS-CoV-2, say scientists at the University of Cambridge.
However, when the E484K mutation – first seen in the South African variant – is added, it substantially increases the amount of antibody required to prevent infection.
The preliminary data also suggest that a significant proportion of over-eighty olds may not be sufficiently protected against infection until they have received their second dose of the vaccine.
As the SARS-CoV-2 virus replicates and spreads, errors in its genetic code can lead to changes in the virus.
Towards the end of 2020, the Cambridge-led COVID-19 Genomics UK (COG-UK) Consortium identified a variant of the virus (now known as B1.1.7) and its emergence led to strict lockdown measures in the UK because of concerns over its transmissibility. There is particular concern that these changes might enable the virus to ‘escape’ the newly-developed vaccines.
The UK has begun rolling out two vaccines – the Pfizer BioNTech vaccine and the Oxford AstraZeneca vaccine. The efficacy of the vaccines can be boosted by a second dose; however, in order to reach as large a number of people as possible in a short amount of time, the government has concentrated on delivering a first dose to as many individuals as possible by giving the second dose at 12 weeks, rather than three.
Researchers at the Cambridge Institute of Therapeutic Immunology & Infectious Disease (CITIID) created a synthetic version of the SARS-CoV-2 virus, known as a pseudovirus. They found that the Pfizer BioNTech vaccine is likely to offer similar protection against B1.1.7 as it does against the previous strain of SARS-CoV-2, however it may be less effective when dealing with E484K mutation, which so far has only been seen in a relatively small number of individuals.
This is an abridged version of the press release which was first published on our website on February 2, 2021.

A DNA test that can identify secondary infections in COVID-19 patients.
Researchers have been able to develop a DNA test to quickly identify secondary infections in COVID-19 patients,
Patients who are diagnosed with severe COVID-19 may need mechanical ventilation in order for clinicians to help treat the virus. However, some may be susceptible to secondary bacterial infections.
COVID-19 patients are thought to be more at risk of a secondary infection because of the amount of lung damage from the virus and will spend more time on a ventilator than those without COVID-19. Many of these patients also have a poorly-regulated immune system, where the immune cells damage the organs and also have impaired anti-microbial functions, so trying to diagnose these patients early is vital.
Cambridge researchers have developed a DNA test to identify those who may have developed the secondary infection a lot sooner.
The test uses multiple polymerase chain reaction (PCR) to help detect the DNA of the bacteria within a few hours rather than waiting for it to grow in the lab. The test runs multiple PCR reactions and can simultaneously pick up 52 different pathogens (organism that causes disease), which often infect the lungs of patients in intensive care. At the same time, it can also test for the bacteria which may be resistant to antibiotics.
Often patients have already started to receive antobiotics before the bacteria has had time to grow meaning cultures are often negative. However, the PCR test doesn’t need to viable bacteria to be able to detect it, not only making it a more accurate test and are able to speed up the diagnosis.
This is one of the first times that this technology has been used in routine clinical practice and was approved by Addenbrooke’s hospital in Cambridge.

Can a tapeworm drug boost protection from COVID-19 for high-risk kidney patients?
UK researchers are launching a clinical trial to investigate if the drug niclosamide, usually used to treat tapeworms, can prevent Covid-19 infection in vulnerable, high-risk kidney patients and reduce the number of people who become seriously ill or die from it.
If the charity and industry-funded trial is successful, it may pave the way for a new treatment to prevent or alleviate the impact of Covid-19 in people on dialysis, people who have had a kidney transplant, and people with auto-immune diseases affecting the kidneys such as vasculitis who require treatment to suppress their immune system. The treatment will last up to nine months.
Patients on the trial will receive either a placebo (or dummy) drug, or UNI911 (niclosamide) as a nasal spray, both provided by the manufacturer UNION therapeutics, in addition to all their usual treatments. The trial plans to expand to other UK healthcare centres and aims to recruit at least 1,500 kidney patients.
Niclosamide has shown real promise in the lab, with early tests showing it could stop SARS-CoV-2 multiplying and entering cells of the upper airways.
Niclosamide has been re-formulated into a nasal spray and participants will take one puff up each nostril twice a day.
The trial will identify whether niclosamide can protect people from the virus either on its own, or in combination with any of the vaccines currently available.
If successful, this trial could mean that the treatment becomes available to kidney patients more widely within months.
This is an abridged version of the press release which was first published on our website on February 22, 2021.

Genomics study identifies routes of transmission of coronavirus in care homes
Genomic surveillance – using information about genetic differences between virus samples – can help identify how SARS-CoV-2 spreads in care home settings, whose residents are at particular risk.
Care homes are at high risk of experiencing outbreaks of COVID-19, the disease caused by SARS-CoV-2. Older people and those affected by heart disease, respiratory disease and type 2 diabetes are at greatest risk of severe disease and even death, making the care home population especially vulnerable.
Care homes are known to be high-risk settings for infectious diseases, owing to a combination of the underlying vulnerability of residents who are often frail and elderly, the shared living environment with multiple communal spaces, and the high number of contacts between residents, staff and visitors in an enclosed space.
In research published in eLife, a team led by scientists at the University of Cambridge and Wellcome Sanger Institute used a combination of genome sequencing and detailed epidemiological information to examine the impact of COVID-19 on care homes and to look at how the virus spreads in these settings.
In this study, researchers analysed samples collected from 6,600 patients between 26 February and 10 May 2020 and tested at the Public Health England (PHE) Laboratory in Cambridge. Out of all the cases, 1,167 (18%) were care home residents from 337 care homes, 193 of which were residential homes and 144 nursing homes, the majority in the East of England. The median age of care home residents was 86 years.
Compared with non-care home residents admitted to hospital with COVID-19, hospitalised care home residents were less likely to be admitted to intensive care units (less than 7% versus 21%) and more likely to die (47% versus 20%).
“Using this technique of ‘genomic surveillance’ can help institutions such as care homes and hospitals better understand the transmission networks that allow the spread of COVID-19,” said Dr William Hamilton from the University of Cambridge and CUH. “This can then inform infection control measures, helping ensure that these places are as safe as possible for residents, patients, staff and visitors.”
This is an abridged version of the press release which was first published on our website on March 3, 2021.

Differing immune responses discovered in asymptomatic cases and those with severe COVID-19
A UK-wide study part-funded by the NIHR has identified differences in people’s immune responses to COVID-19, depending on whether they have no symptoms or more serious reactions to the virus.
In the study, researchers and their collaborators in the Human Cell Atlas initiative analysed blood from 130 people with COVID-19. These patients came from three different UK centres in Newcastle, Cambridge and London and ranged from asymptomatic to critically severe.
The researchers found raised levels of specific immune cells in asymptomatic people to help fight infection – but patients with more serious symptoms had lost these protective cell types and instead gained inflammatory cells. In severe cases this led to lung inflammation, blood clotting difficulties and hospitalisation.
While it is not yet understood how the infection stimulates these immune responses, the study gives a molecular explanation for how COVID-19 could cause an increased risk of blood clotting and inflammation in the lungs, which can lead to the patient needing a ventilator.
This also uncovers potential new therapeutic targets to help protect patients against inflammation and severe disease.
In the future, research may identify those who are more likely to experience moderate to severe disease by looking at levels of these immune cells in their blood.
This is an abridged version of the press release which was first published on our website on April 22, 2021.
Cambridge hosts world-first COVID-19 vaccine booster study
The Cov-Boost study offered individuals a chance to have a third dose of COVID-19 vaccine to see whether such a booster dose can better protect against the virus.
In this Government-funded trial, Cambridge were one of the sites to host the ‘booster’ COVID-19 vaccine trial at the NIHR Cambridge Clinical Research Facility.
With thousands of volunteers taking part in the UK, the study would provide researchers vital data on the impact of a third dose on patients’ immune responses.
The trial looked at seven different COVID-19 vaccines (including the Pfizer/BioNTech, and Valneva vaccines) as potential boosters, given at least 10 to 12 weeks after a second dose as part of the ongoing vaccination programme. One booster will be provided to each participant and could be a different brand to the one they were originally vaccinated with.
All the data was then analysed to help inform decisions by the Joint Committee on Vaccination and Immunisation (JCVI) on any potential booster programme for autumn of 2021.
Researchers in Cambridge saw more than 180 participants from the Cambridgeshire area. Professor Krishna Chatterjee, Director of the NIHR Clinical Research Facility in Cambridge, who led the trial in Cambridge said in June: “We are delighted to support this study here in Cambridge. We have conducted trials of several COVID-19 vaccine studies over the last year. It’s an exciting opportunity to now work on a study to determine the effects of a third ‘booster’ dose of vaccines and I want to thank both the trial participants and our staff who are helping with this important research.”
Read the full story from June 2021.
Key mutations in Alpha variant enable SARS-CoV-2 to overcome evolutionary weak points
One of the key mutations seen in the ‘Alpha variant’ of SARS-CoV-2 – the deletion of two amino acids, H69/V70 – enables the virus to overcome chinks in its armour as it evolves, say an international team of scientists.
SARS-CoV-2 is a coronavirus, so named because spike proteins on its surface give it the appearance of a crown (‘corona’). The spike proteins bind to cells in our body, where the virus then replicates and spreads.
But as it divides and replicates, it also mutates. Some mutations make the virus more infectious, some help it evade the immune response, potentially making vaccines less effective, while others have little effect.
Towards the end of 2020, Cambridge scientists observed SARS-CoV-2 mutating in the case of an immuno-compromised patient treated with convalescent plasma (where the patient received blood plasma which already had antibodies). In particular, they saw the emergence of a key mutation – the deletion of two amino acids, H69/V70.
This deletion has since been seen across much of Europe, Africa and Asia – the so-called ‘Alpha’ variant – and appears to have spread multiple times independently.
Working under secure conditions, Professor Gupta and colleagues used a ‘pseudotype virus’ to understand how the spike protein interacts with host cells and what makes this mutation so important.
They found that the deletion makes the virus twice as infectiv – that is, the variants were both better at escaping immunity and more infectious.
This is an abridged version of the press release which was first published on our website on June 29, 2021.
‘Biological fingerprint’ in blood could help identify COVID patients with no symptoms
Cambridge researchers have discovered a biomarker – a biological fingerprint – in the blood of patients who previously had COVID-19.
This means they can identify people who have had COVID-19 even if they displayed no symptoms – and the biomarkers last several months after infection.
Current practice requires people to take a PCR test at the time of infection or an antibody test, to see if they had the virus but were asymptomatic.
As a result of the research the team has received £370,000 from the National Institute for Health Research (NIHR) to develop a COVID-19 diagnostic test that will complement existing antibody tests, as well as develop a test that could diagnose and monitor long Covid.
The research builds on a pilot project supported by the Addenbrooke’s Charitable Trust which has been recruiting patients from the Long COVID Clinic established in May 2020 at Addenbrooke’s Hospital.
During the pilot, the team recruited 85 patients to the Cambridge-led NIHR COVID BioResource, which collects blood samples from patients when they are first diagnosed and then at follow-up intervals over several months.
In their initial findings, they identified a molecule known as a cytokine produced by T cells in response to infection – which persists in the blood for a long time after infection.
By following patients for up to 18 months post-infection, the team hopes to address several questions, including whether immunity wanes over time. This will be an important part of helping understand whether people who have been vaccinated will need to receive boosters to keep them protected.
As part of their pilot study, the team also identified a biomarker found in patients with long COVID. Their work suggests these patients produce a second type of cytokine, which persists in patients with long COVID and might be useful for diagnosing long COVID and help in the development of new treatments against COVID.
This is an abridged version of the press release which was first published on our website on July 19, 2021.

World first for AI and machine learning to treat Covid patients worldwide
In a ground-breaking study supported by the NIHR Cambridge BRC, Addenbrooke’s Hospital, healthcare technology firm NVIDIA and 20 other hospitals worldwide have used artificial intelligence (AI) to predict Covid patients’ oxygen needs.
In what’s known as federated learning, the research applied an algorithm to analyse anonymised electronic patient health data and chest x-rays from 10,000 Covid patients worldwide, including 250 at Addenbrooke’s Hospital.
The study – dubbed EXAM – took just two weeks of AI ‘learning’ to achieve high-quality predictions on how much extra oxygen a patient would need in the first days of hospital care.
To maintain strict patient confidentiality, the patient data was fully anonymised and an algorithm was sent to each hospital so no data was shared or left its location.
Once the algorithm had ‘learned’ from the data, the analysis was brought together to build an AI tool which could predict the oxygen needs of hospital Covid patients anywhere in the world.
This model can be used to help frontline physicians worldwide.
This is an abridged version of the article first published on our website on 15 September 2021.
Positive phase 3 results reported in trial for new COVID-19 vaccine supported by Cambridge
A clinical trial supported by the NIHR Cambridge BRC and NIHR Cambridge CRF for a new vaccine against COVID-19 has received positive Phase 3 results.
The trial has been taking place at 22 locations across the UK and recruited a total of 4012 participants aged 18 years and over, and 660 adolescents.
Results showed in October 2021, that the vaccine was successful in producing high levels of neutralising antibodies against the COVID-19.
Developed by the French specialty vaccine company Valneva and manufactured in Scotland, the vaccine is the only inactivated, adjuvanted COVID-19 vaccine in clinical development in Europe. This means, that like flu and polio vaccines, it contains dead versions of the virus that cannot cause disease. Valneva hopes to initially get the jab approved for those aged between 18 and 55.
This national trial was supported at the Cambridge site by the NIHR Cambridge Clinical Research Facility and NIHR Cambridge BRC.


