Patient and Public Involvement, Engagement and Participation (PPIE) Strategy 2022-2027


This Strategy is intended to be read and delivered in parallel with the NIHR Cambridge BRC and NIHR Cambridge CRF Joint Equality, Diversity and Inclusion Strategy.
This is a joint strategy from the NIHR Cambridge Biomedical Research Centre and NIHR Cambridge Clinical Research Facility. Open this link for an accessible word document of the strategy. Click on the bookmarks below if you wish to jump to a particular section within the strategy.
Introduction
Definitions
Purpose
Key Objectives
Schedule of Activities (this links to a child page)
Resources
Governance, Reporting and Leadership
Monitoring Success and Capturing Impact
Partners and Collaborators
Introduction
Patients and public are at the heart of the research that we do on the Cambridge Biomedical Campus. The National Institute for Health and Care Research (NIHR) Cambridge Clinical Research Facility (CRF) will work together with the NIHR Cambridge Biomedical Research Centre (BRC) to deliver joined-up patient and public engagement and involvement with partners and collaborators across the East of England. Our priority is to ensure that patients and the local and wider community are involved in all aspects of work the research that we do.
This joint strategy has some activities that cross over between the BRC and CRF. However our action plan clearly states who is responsible for each PPIE activity and objective.
Definitions
The words describing patient and public involvement, engagement (PPIE) as well as the word ‘participation’ can be confusing. We use the NIHR definitions throughout this document:
Involvement: Where members of the public are actively involved in research projects and in research organisations and research is carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them
Engagement: Where information and knowledge about research is provided and disseminated
Participation: Where people take part in a research study
Purpose
Through our Patient and Public Involvement, Engagement and Participation (PPIE) strategy, we will build long-term partnerships across our region to support shared ownership and mutual learning between researchers and communities, identify research questions relevant to those communities and drive translation of scientific discoveries into improved health care. We believe that meaningful involvement in and engagement will make our research more relevant, more accessible to patients and more likely to make a positive difference to improving healthcare and reducing health inequalities.
We aim to build collaborations with patients and partners to increase involvement, engagement and participation in research, with a particular emphasis on underserved communities.
Key Objectives
Inclusive research starts with Patient and Public Involvement and Engagement (PPIE) at all stages of the research cycle. PPIE and equality, diversity and inclusion (EDI) are embedded across our BRC and CRF – from our governance structure through to the specific objectives of our research and core themes, in addition to the objectives laid out in this strategy.
We aim to go out into local communities to engage with new communities and raise research awareness and to provide a range of accessible involvement opportunities to expand the number and diversity of groups that get involved in our research.
Our objectives will be reviewed annually during this during this 5-year period to align with changes over time.
We have 5 key objectives for our Joint PPIE strategy:
- The questions we research are the ones that matter to our patients and communities
- Our PPIE activities are rooted in the research that we do
- Our research design and processes are inclusive
- PPIE opportunities are accessible to everyone in our region
- Results and impact are shared with participants, collaborators and stakeholders
These goals have been developed in collaboration with our PPIE working groups within the BRC and CRF, and with our public contributors through the Cambridge University Hospitals (CUH) Patient and Public Involvement Panel.
Schedule of Activities 2022-2027
Action Plan
The overall goal of the Patient and Public Involvement, Engagement and Participation (PPIE) strategy is to ensure that patients and the public are involved and engaged at all stages of our research and that we deliver meaningful PPIE to a high standard.
We have developed and agreed these PPIE key objectives and associated actions with reference to the NIHR UK Standards for Public Involvement, which were designed to improve the quality and consistency of public involvement in research.
The schedule of actions and activities the NIHR Cambridge BRC and NIHR Cambridge CRF will undertake to achieve our SMART objectives is outlined here.
Resources
NIHR Cambridge BRC
Our BRC has a PPIE/communications support team (4.6 FTE), including a PPIE Lead (Dr Amanda Stranks), who support central PPIE activities, advise researchers on their PPI plans and drive our communication and dissemination strategy. The core budget supports payment and reimbursement of our CUH PPIE panel, through which we provide an inclusive range of PPI opportunities open to all across our region, our PPIE training programme for researchers and contributors, and seed funding for PPIE activities. We are also investing in a new role of ‘Inclusive Research Lead’ (band 7, 1.0 FTE) to support our ambitious plans to improve EDI across our research. The inclusivity lead will work alongside our PPIE team, PPIE Champions and regional partners to build long-term relationships with diverse and historically excluded communities.
The CUH PPI panel currently has around 70 members, and is open to anyone across the East of England who is interested in getting involved in research. Researchers at the BRC and CRF are able to involve the panel in their research projects, with the costs for this involvement covered centrally through our PPI budget. The PPIE team also support researchers to directly recruit public contributors with relevant lived experience to be involved in research projects and funding applications supported through the BRC and CRF.
In collaboration with our Training and Development Team, we provide both introductory and more targeted PPI training to researchers across Cambridge. Our core offering currently covers topics such as PPIE activities, planning inclusive PPIE and capturing and sharing impact of PPIE. All sessions have been co-developed with our public members and involve a public contributor in their delivery. We also provide support to our investigators to design induction and training for their public contributors.
We support our public contributors through biannual meetings to review progress against our strategy and co-design activities for the following 6 months. Contributors are invited to build their skills through our research information sessions, which cover topics that arise frequently across our research projects (such as Artificial Intelligence, the role of the Research Ethics Committee and how medical devices are regulated) or as requested by our public members.
We will also fund themes to use Thiscovery, an innovative digital research platform designed by The Healthcare Improvement Studies Institute in Cambridge to enable coalitions of patients, community groups and researchers to come together through online engagement/involvement activities. The platform is being used successfully by our themes working with contributors who sometimes struggle with physical meetings, including frail elderly people and their carers, the mentally ill and people living with obesity.
NIHR Cambridge CRF
Our CRF funds a dedicated PPIE Lead (Caroline McMahon), Communications specialist (Band 6×0.2) and Staff PPI champions for each CRF unit. The research champions deliver programs of work aligned to our PPI strategy. In addition, members of the wider CRF team help with delivery of PPIE activities on an ad hoc basis.
The CRF also resources the Participant Survey, allowing participants in research taking place at the Clinical Research Facility to share their experiences of participation.
Governance, Reporting and Leadership
The BRC and CRF have internal governance structures and lines of reporting, reporting to our host organisation, Cambridge University Hospitals NHS Foundation Trust and our key partner, the University of Cambridge. This is strengthened by co-opted members from the BRC and CRF within each other’s governance structures and through public membership on key decision-making committees.
The individual and joint governance arrangements of the NIHR Cambridge BRC and CRF are outlined in the organogram and table below. The tables are colour-coded to illustrate how they link with the organogram, and to highlight cross-organisational membership to promote strategy alignment, prevent duplication and share best practice.
The organogram and table are also available separately in an accessible word-document.
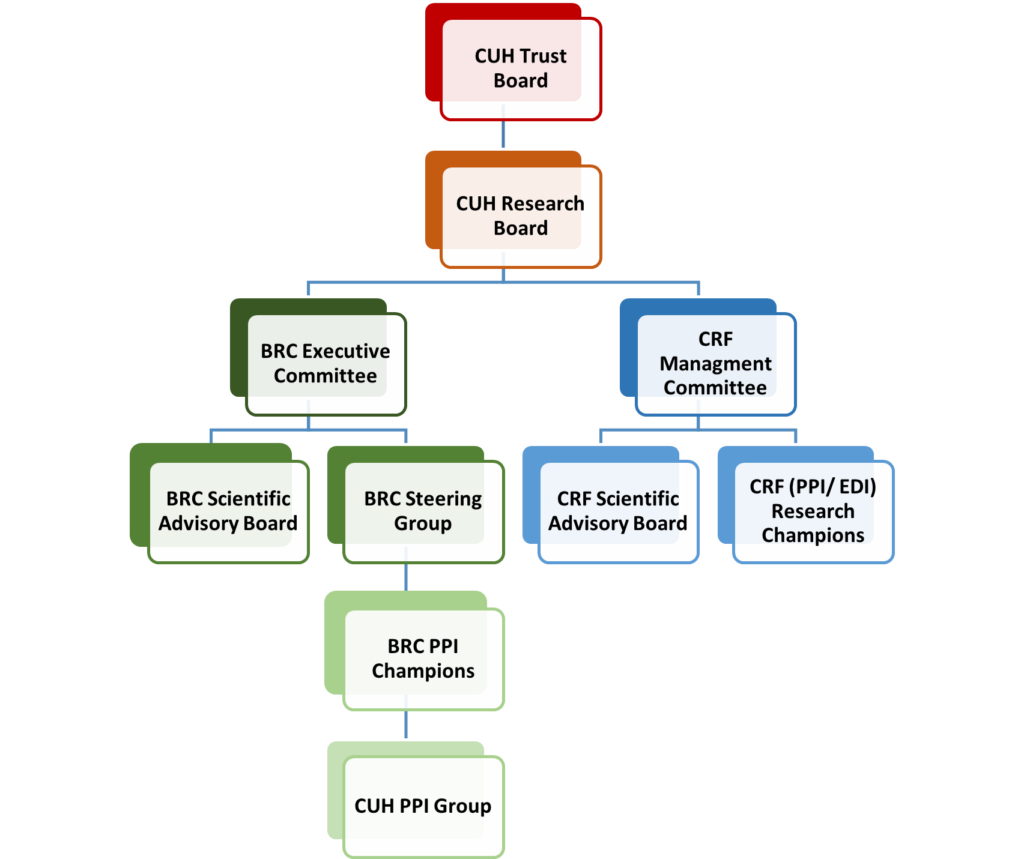

CUH Research Board
CUH Executive Director for Research
CUH Director of Finance
CUH R&D Finance Director
CUH Director of Research
CUH Chief Nurse
Professor of Nursing Research
CUH Chief Pharmacist
BRC Director
CRF Director
Clinical Directorate Academic Leads
Director Clinical Trials Unit

BRC Executive Committee
BRC Director
Regius Professor of Physic (University of Cambridge)
CUH Director of Research
CUHP CEO
Director of Organisational Affairs (University of Cambridge, School of Clinical Medicine)
CRF Director
Director of The Healthcare Improvement Studies Institute
Two public members

BRC Steering Group
BRC Director
Regius Professor of Physic (University of Cambridge)
BRC Scientific Director
BRC Capacity Building Lead
BRC Research theme leads/co-leads
BRC PPI Lead
CRF Director
Director NIHR Applied Research Collaborative (East of England)
Wellcome Genome Campus representative
NIHR BioResource Director
Chair of Nursing Research

BRC PPI Champions
BRC director
BRC PPI Lead
PPI champions from each BRC research them
CRF PPI Lead
BioResource PPI Lead

CRF Management Committee
CRF Director
CRF co-director
Director of Operations
Director of HLRI
CRF manager x 2
Matrons x 3
Workforce development lead
CRF PPI Lead
Operations manager
Quality assurance manager
Experimental medicines and industry lead
Ward team leaders

CRF PPI/EDI Research Champions
CRF PPI Lead
Representative from each CRF Unit (x7)
CRF Public member
HLRI Public member
The CRF PPIE Forum / CRF PPI / EDI Research Champions meet quarterly to review progress against objectives, record impact and provide reports for the CRF Patient Safety and Governance Committee, which meets quarterly.
Leadership and governance of national collaborations with the UKCRF Network PPIE Workstream is provided by the Workstream Chair and UKCRF Network Lead and is aligned to the NIHR UKCRF Network funded programme of work. Public and PPI Lead representation on the CRF Scientific Advisory Board and Management Committee provide public oversight.
PPIE at the BRC is led by the director, with the support of the PPIE Lead. It is a standing item on the BRC Steering Committee agenda (attended by all theme leads/co-leads), and the BRC Executive Committee, which also has two public members, supported by the BRC PPI lead. All BRC themes and senior investigators are integrally involved in the PPIE work plan, each has a named PPIE Champion on their leadership team, personnel funded to support their PPIE activities and PPIE objectives relevant to the delivery of research within their themes. The BRC Director meets twice yearly with theme PPIE champions, together with the PPIE Lead, enabling dissemination of learnings across the BRC, CRF and our wider community.
Progress against BRC PPIE objectives will be monitored as part of the BRC Director’s annual theme appraisal, and our PPIE lead will report outcomes formally to the BRC Executive Committee and the CUH PPI panel biannually. We will also undertake an independent, formal qualitative review of our joint PPIE strategy at the mid-point of the cycle to assess the impact of our strategy, and incorporate findings into our practice.
Public contributors also play an integral role in our governance and accountability to our objectives through involvement in our governance.
Public & patient involvement in CRF governance structures:
- New Executive Board with strategic oversight of all CRFs/Units in CCRC at CUH & RPH, will include two public/patient members.
- Scientific Advisory Board which reviews all CCRC studies, will include two public & patient members plus PPIE Leads.
- Management Committee, reviewing CRF operations, will include public & patient members.
Public & patient involvement in BRC governance structures:
- BRC Executive Committee with strategic oversight of all BRC activity and decision making has two public/patient members
- The Health Data Access Committee has a lay member
Monitoring Success and Capturing Impact
The PPI Leads from the CRF and the BRC will lead on monitoring the success of our PPI strategy and work with PPI champions and investigators across our infrastructure to ensure that impact from PPIE is captured and shared.
If we are successful in our objectives, we expect to see increased relationships with communities and patient groups across our region, increased influence from our stakeholder groups in the setting of priorities for our research and more regular discussion and sharing of impact and findings of our PPI as part of our routine research delivery.
To meet our objective of ensuring that results and impact are captured and shared, we will co-develop a set of suggested questions for identification and capture of impact and reflective evaluation of PPI activity. We will also use this framework to create a template for researchers to create shareable case studies of how PPIE has influenced their research and feature these on our website, in our PPI newsletter and at our community events.
Partners and Collaborators
In addition to partnering with each other to deliver the objectives outlined in this strategy, the BRC and CRF work closely with other health, research and community organisations based on the Cambridge Biomedical Campus (CBC) and across the East of England. We believe that working together will lead to greater impact, and make opportunities for involvement more accessible and inclusive.
The Cambridge University Health Partners PPI forum unites the PPIE Leads of NHS and research organisations based on the CBC, including those from the Royal Papworth Hospital and Cambridge and Peterborough Foundation Trust and representatives from patient governors at each of CUHPs constituent hospitals. We meet twice yearly to align and harmonise our PPIE activity, identify shared challenges and opportunities and escalate shared issues to enable a united approach.
We coordinate our PPIE activities with aligned NIHR infrastructures (Clinical Research Network, Applied Research Collaboration-East, BioResource) across our region through the East of England PPI collaborative, which meets quarterly to leverage resources and ensure that our approach is coordinated and cohesive. This will include shared engagement events for areas of common interest such as dementia and mental health.
The BRC has also formed a partnership with the PPIE team and PPI-aligned academics from the University of East Anglia, to grow our combined community networks and leverage our resources. Together we host an annual Regional PPI week of activities and are forming partnerships between academics and themes with shared interests to plan shared engagement events.
We also regularly communicate with aligned community and health organisations, such as Healthwatch and our regional Integrated Care Boards to share opportunities across our combined community networks and support each other’s community events.


