Devices and Advanced Therapies


This theme’s focus:
- Creating trusted medical devices from algorithms and mobile apps
- Transforming implants and bio-interfaces for diagnosis and monitoring
- Generating innovative delivery platforms for advanced therapies
- Developing advanced cellular, stem cell and gene therapies
The Devices and Advanced Therapies theme was developed in line with the UK government’s Life Sciences Vision 2021, which aims to fast-track patient access to new treatments and technologies.
This is an exciting new theme driven by the need to support the rapid advancements in Medical Devices and Advanced Therapy research in Cambridge.
The term ‘medical device’ includes a broad range of products that are used in healthcare. They can be physical items (such as a blood pressure monitor) or software (such as a healthcare app on your phone) which are used for the diagnosis, prevention, monitoring or treatment of illness or disability.
The term ‘advanced therapies’ covers medicinal products that are either gene therapies, cell therapies or tissue-engineered therapies. These are designed to treat underlying conditions.
Our clinicians and researchers have the world-leading expertise to develop new devices and therapies, and this theme will facilitate and accelerate the pathway from small-scale lab-based developments through to providing benefit for patients in clinical practice.
We will use the NIHR/MRC Framework for developing and evaluating complex interventions to support these pathways.
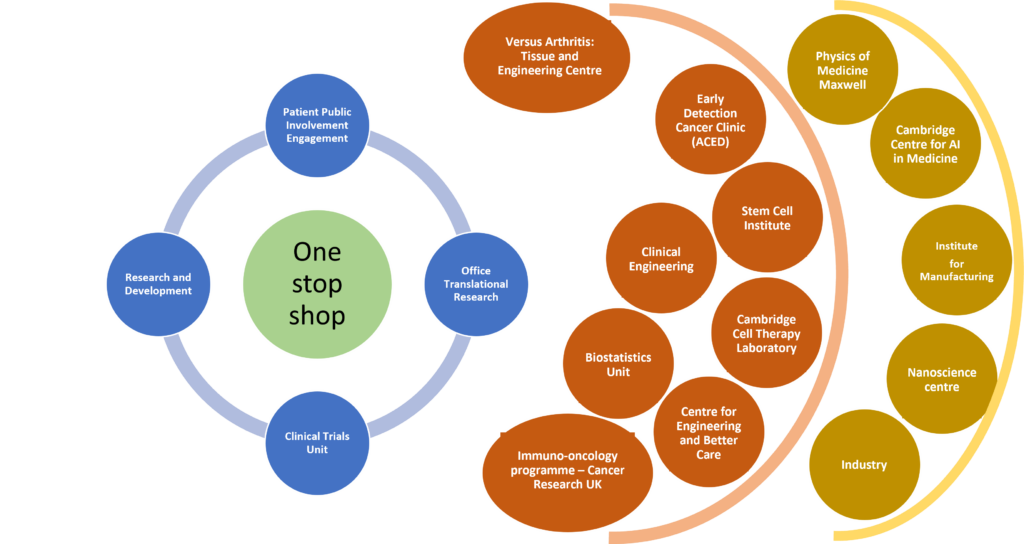
We have a ‘One Stop Shop’ to support and coordinate the pipeline from idea to proof of concept, real-world testing, adoption and roll-out in the NHS. This figure shows our network of resources to support development pathways.
We support our innovations and future researchers with our ‘pump-priming’ funding, led by our emerging-leaders team.
Our Patient and Public Involvement and Engagement (PPIE) group will help us to close the gap between proof of concept and implementation (routine use) by ensuring that our research is meaningful and relevant for patients and members of the public and enabling us to improve transparency and awareness. We will run public activities to involve patients in panel discussions, provide regular updates on the use of patient data and facilitate access to Devices and Therapies trials.
Our PPIE champion, Dr Bhavisha Parmar, will work with our researchers to engage the young minds of pupils and families through an outreach programme in schools and colleges across our region. In the video below Dr Parmar talks about the BEARS project (Both Ears Training Package) for children with cochlear implants.

