Who has reviewed this trial?
This trial has been reviewed and given favourable opinion by North West – Greater Manchester South Research Ethics Committee.
The Medicines and Healthcare Products Regulatory Agency (MHRA) who are responsible for regulating medicines in the UK have also reviewed this trial.
Who is funding the trial?
The Evolution Education Trust funds education and research projects in the science of genetics, biodiversity and the Principles of Evolution, including development and delivery of therapeutic solutions for a public health benefit.
What will happen to the results of the trial?
When the results of this trial are available, they may be published in peer reviewed medical journals and used for medical presentations and conferences.
They will also be published on the EU Clinical Trials Register website, a central registry for all clinical trials conducted in the EU.
Anonymous datasets from the trial may also be made available to other researchers in line with national and international data transparency initiatives.
If you would like to obtain a copy of the published results, please contact your trial doctor directly who will be able to arrange this for you.
Genetic Tests
DNA is the chemical that makes up genes, influencing the factors we inherit and which determine our characteristics.
We will also isolate and test other components of your blood such as RNA and protein and measure chemicals in the blood.
We hope the results of this profiling will help us understand COVID-19 better. As this research is exploratory, you will not receive feedback regarding any ‘markers’ identified in your DNA.
What will happen to my samples?
During the trial we will analyse your samples in a central laboratory. With your permission any unused samples at the end of this trial will be stored for future tests related to this study, and for future approved research projects.
Will my taking part in this trial be kept confidential?
They will be using information from you and your medical records in order to undertake this trial and will act as the data controller for this trial. This means that they are responsible for looking after your information and using it properly.
The Sponsor organisation(s) will keep identifiable information about you for 5 years after the trial has finished to ensure your safety and allow the trial to be reviewed by the authorities after it is finished.
Your rights to access, change or move your information are limited, as the Sponsor organisation(s) need to manage your information in specific ways in order for the research to be reliable and accurate. To safeguard your rights, we will use the minimum personally-identifiable information possible.
You can find out more about how the Sponsor(s) use(s) your information through the weblink and email address below.
Cambridge University Hospitals will collect your name, NHS number and contact details to contact you about this trial, and make sure that relevant information about the trial is recorded for your care, and to oversee the quality of the trial.
Individuals from the Sponsor(s) and regulatory organisations may look at your research records to check the accuracy of this trial. Cambridge University Hospitals will pass these details to the Sponsor(s) along with the information collected from you. The only people in the Sponsor organisation(s) who will have access to information that identifies you will be people who need to contact you in relation to this trial and to audit the data collection process.
All information collected about you as a result of your participation in the trial will be kept strictly confidential. Your personal and medical information will be kept in a secured file and be treated in the strictest confidence.
Once you have agreed to participate in this trial you will be allocated a Trial ID Number. This is a unique trial number which will be used on all your trial documentation along with your date of birth. Your date of birth is considered to be personal information. We collect this personal information on trial documentation to help ensure that the data we receive as part of your trial participation is correctly allocated to you. By cross-checking these two unique references we can ensure the integrity of the data.
The people who analyse the information will not be able to identify you and will not be able to find out your name, or contact details. Only anonymous trial data, without
When you agree to take part in this trial, the information about your health and care may be provided to researchers running other research studies in this organisation and in other organisations. These organisations may be universities, NHS organisations or companies involved in health and care research in this country or abroad. Your information will only be used by organisations and researchers to conduct research in accordance with the UK Policy Framework for Health and Social Care Research.
This information will not identify you and will not be combined with other information in a way that could identify you. The information will only be used for the purpose of health and care research and cannot be used to contact you or to affect your care. It will not be used to make decisions about future services available to you, such as insurance.
We will need to inform your GP of your participation in this trial so that any medical decisions made by your GP account for any treatment you are receiving as part of this trial. We may also request information from your GP, such as a discharge summary should you be hospitalised with COVID-19.
What if there is a problem?
If you have any concerns about any aspect of this trial you should speak to your trial doctor who will do their best to answer your questions.
In the event that something does go wrong and you are harmed by taking part in the research and this is due to someone’s negligence then you may have grounds for a legal action for compensation against Cambridge University Hospitals NHS Foundation Trust.
If your claim is successful, your legal costs will be met. The normal National Health Service complaints mechanisms will still be available to you (if appropriate).
The NHS does not provide no-fault compensation i.e. for non-negligent harm, and NHS bodies are unable to agree in advance to pay compensation for non-negligent harm. They are able to consider an ex-gratia payment in the case of a claim.
If you wish to complain or have any concerns about any aspect of the way you have been approached or treated during this trial, you can do this through the NHS complaints procedure. In the first instance it may be helpful to contact the Patient Advice and Liaison Service (PALS) at your hospital.
What if I decide I no longer wish to participate in the trial?
If you decide not to participate any further, you will no longer receive the trial treatment. No further tests will be performed on you and no further research samples will be collected.
Any data already collected or results from tests already performed on you or your samples will continue to be used in the trial analysis.
The trial doctor may also choose to withdraw you from the trial if they feel it is in your best interests or if you have been unable to comply with the requirements of the trial.
Reasons for trial withdrawal could include:
- You have experienced a serious side effect
- You are unable to complete the visits, medication or trial documentation as required
If you have experienced any serious side effects during the course of the trial which require you to withdraw from the trial, your trial doctor will follow up with you regarding your progress until the side effect has stabilised or resolved.
What if new information becomes available?
Your trial doctor will contact you to discuss the new information and whether you wish to continue participating in the trial.
If you still wish to continue on the trial, you will be asked to sign a new Informed Consent Form.
The trial sponsor, the regulatory authority or the trial doctor may decide to stop the trial at any time. If that happens, we will tell you why the trial has been stopped and arrange for appropriate care for you.
Expenses & Payment
What happens when the trial stops?
Once the trial has ended the study drugs may not be available to healthcare workers. However, pending the results of the trial, treatment guidelines may change.
What are the alternatives for treatment?
What are the possible benefits of taking part?
However, information collected as part of your participation in this trial may benefit patients with COVID-19 or healthcare workers or other individuals who come in contact with COVID-19 patients in the future.
What are the possible disadvantages and risks of taking part?
However, individual patients can react differently to the same drug which means that there is a possibility of experiencing side effects.
In addition, if you are diabetic and taking medication for your diabetes, there is a small risk of hypoglycaemia during the 2-day loading phase of the trial.
You will be encouraged to monitor your blood glucose levels carefully during this period, and to consume a bed-time snack if possible. The trial doctor will monitor any side effects regularly and take appropriate actions where necessary.
What are the side effects of Hydroxychloroquine?
As such, side effects may be less likely to occur and are unlikely to result in significant adverse events. These risks will be assessed in every participant.
Common (less than 10% of patients):
- abdominal pain
- decreased appetite
- diarrhoea
- feeling sick
- vomiting
- mood changes
- skin rash
Uncommon (less than 1% of patients):
- hypoglycaemia (low blood sugar)
- loss of hair (alopecia)
- worsening of eyesight
- blurred vision
- double vision anxiety
- leg or arm weakness
- tingling or numbness
- seizures
- ringing in the ear (tinnitus)
- feeling dizzy
- sensation of unsteadiness or spinning (vertigo)
- low blood or platelet count
Hydroxychloroquine is also associated with other potential ophthalmic side effects including reduced visual acuity, reduced peripheral vision and nocturnal visual impairment.
However, these side effects are associated with prolonged use of hydroxychloroquine (>5 years) and the Royal College of Ophthalmologists advise annual screening for retinal disease for treatment durations of 5 years or longer.
There is also a risk of QT prolongation associated with hydroxychloroquine use, but this risk is extremely low when the duration of hydroxychloroquine treatment is short-term (as in the current trial) and at the doses used in this trial.
What will I have to do?
Between hospital visits you will need to complete some brief questions remotely via telephone/online/web-based interface on a weekly basis. You should continue to use PPE as recommended by PHE and your hospital guidelines at all times during this study.
It is important that you take any trial medication regularly as directed by your trial doctor. You will be given 3 bottles of identical medication with instructions on when to take doses from each bottle.
It is important that you follow these instructions carefully to ensure that you are taking the correct medication at the correct interval. This will occur for the loading and maintenance phases.
On visit 2 you will receive the remainder of the trial medication with instructions to complete the maintenance phase. Trial medication should be taken at roughly the same time each day.
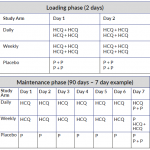 The table below shows dosing regimes for both the loading phase and maintenance phase of the trial. (Click on the image to enlarge.)
The table below shows dosing regimes for both the loading phase and maintenance phase of the trial. (Click on the image to enlarge.)
Administration of medication:
Each dose should be taken with a meal or glass of milk and tablets swallowed whole.
Missed or replacement doses:
Do not replace vomited doses. Do not make up for missed doses by taking extra tablets if more than 12hrs has elapsed after the time it/they would usually be taken. In the event of missed doses, do not double the number of tablets to make up the missed dose.
Any unused study treatment must be returned to the study site.
Permitted and prohibited medications:
Over-the-counter medications such as paracetamol (doses <2.0g/day) are permitted. Grapefruit and/or grapefruit juice should be avoided. Antacids should be avoided within ~4 hours of dosing. You should tell the trial team if you feel unwell or different in any way. If you have any major concerns or are feeling very unwell please contact your trial doctor immediately using the contact numbers below. If you experience potential COVID-19 symptoms or become NHS COVID test positive/COVID positive during the trial you should contact the trial doctor immediately and stop taking your trial medication. You should discuss your participation in this trial with any insurance provider you have (e.g. protection insurance, life insurance, income protection, critical illness cover and private medical insurance) and seek advice if necessary, as failure to notify them may affect or invalidate your cover.
What will happen to me if I take part?
As we don’t know whether Hydroxychloroquine can reduce the risk of healthcare workers contracting the coronavirus, or whether the dose of Hydroxychloroquine as a prevention treatment is important, we need to compare the treatment (Hydroxychloroquine) with Placebo in different dosing regimens.
There are 3 groups in this study and each group will receive a different treatment. You will be allocated one of the groups for this trial in a random way (by chance) using a computer algorithm. You will have a 3:3:2 chance of receiving either:
- Hydroxychloroquine daily (loading dose of 2 x 200mg twice daily for first two days (800mg/day), then dosed daily; 200mg/day) plus weekly placeboli;
- Hydroxychloroquine weekly (loading dose of 2 x 200mg twice daily for first two days (800mg/day), then dosed weekly; 400mg/week) plus daily placebo; or
- Placebo (loading dose of 2 tablets twice daily for first 2 days, then dosed daily with an additional 2 tablets weekly).
Below is a list of the assessments and procedures that we will carry out in the trial. Some assessments rely on local logistics and/or NHS resources and if not undertaken will not affect your participation in the trial. These include COVID-19 tests, respiratory screens and blood samples across visits 1-3, as outlined below:
- Visit 1: Baseline (duration, approximately 45 minutes at hospital): Where possible, we will perform a standard COVID-19 test/s (this may not be analysed until a later date due to current limitations in obtaining results) and respiratory screen for common respiratory viruses. These will be taken by nasal and throat swab, or blood, as per current clinical practice guidelines at each visit. We will ask you about your medical history, concomitant medications, and current work setting.
You will also be invited to give a blood sample (blood group, serum and genetic store; the maximum amount of blood taken will be approximately 30ml [2 tablespoons] at each visit).
We shall then explain how to complete some simple / brief questions to monitor your health weekly. These can be completed over web/app-based interfaces or telephone.
Following this, you will be randomised to a treatment group and given your treatment pack containing your trial medication for the first 7 weeks.
- Visit 2: Interim> (duration, approximately 45 minutes at hospital): This will take place approximately 6 weeks after baseline Visit 1. You should not take your trial medication on the morning of this visit. Where possible, we will perform a second COVID-19 test/s and respiratory screen as before.
We will review your current work setting, concomitant medications, and ask about any adverse events you may have experienced. You will also be invited to give another blood sample (drug levels and serum store).
- Visit 3: End of study (duration, approximately 45 minutes at hospital): This will take place once you have completed your trial medication, approximately 13 weeks after baseline visit 1.
Where possible, we will perform a final COVID-19 test/s and respiratory screen. We will review your current work setting, concomitant medications and ask about any adverse events. You will be invited to give a final blood sample (serum store).
- Weekly assessments (duration, approximately 5-10 minutes performed remotely via telephone or web/app interface): These will be undertaken weekly for 13 weeks, 2 of which will be conducted during hospital visits 2 and 3.
We shall ask you to confirm you have taken your medication over the preceding week, and report any new or review any ongoing adverse events. We will also ask you to complete brief questions to assess your symptoms, self-reported recovery and incidence of health care usage.
Do I have to take part?
If you decide to participate you will be asked to sign an Informed Consent Form, however you are still free to change your mind and leave the trial at any time without giving a reason. If you choose not to participate or to leave the trial, your future medical treatment will not be affected in any way.
Why have I been invited?
We believe Hydroxychloroquine may offer protection against developing symptoms/contracting the virus. We plan to include 1,200 participants who are NHS frontline healthcare workers from at least 3 hospitals across the UK.
What is the drug being tested?
Hydroxychloroquine is commonly used to treat malaria and rheumatological conditions and its side effects are well-known. The doses used in this trial are at the lower end of the range of normal treatment doses prescribed for the conditions they are licensed for, so side effects may be less likely to occur.
This is a double-blind trial meaning neither you nor the trial doctor will know which treatment you have been assigned to.
