What will I have to do?
Between hospital visits you will need to complete some brief questions remotely via telephone/online/web-based interface on a weekly basis. You should continue to use PPE as recommended by PHE and your hospital guidelines at all times during this study.
It is important that you take any trial medication regularly as directed by your trial doctor. You will be given 3 bottles of identical medication with instructions on when to take doses from each bottle.
It is important that you follow these instructions carefully to ensure that you are taking the correct medication at the correct interval. This will occur for the loading and maintenance phases.
On visit 2 you will receive the remainder of the trial medication with instructions to complete the maintenance phase. Trial medication should be taken at roughly the same time each day.
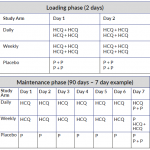 The table below shows dosing regimes for both the loading phase and maintenance phase of the trial. (Click on the image to enlarge.)
The table below shows dosing regimes for both the loading phase and maintenance phase of the trial. (Click on the image to enlarge.)
Administration of medication:
Each dose should be taken with a meal or glass of milk and tablets swallowed whole.
Missed or replacement doses:
Do not replace vomited doses. Do not make up for missed doses by taking extra tablets if more than 12hrs has elapsed after the time it/they would usually be taken. In the event of missed doses, do not double the number of tablets to make up the missed dose.
Any unused study treatment must be returned to the study site.
Permitted and prohibited medications:
Over-the-counter medications such as paracetamol (doses <2.0g/day) are permitted. Grapefruit and/or grapefruit juice should be avoided. Antacids should be avoided within ~4 hours of dosing. You should tell the trial team if you feel unwell or different in any way. If you have any major concerns or are feeling very unwell please contact your trial doctor immediately using the contact numbers below. If you experience potential COVID-19 symptoms or become NHS COVID test positive/COVID positive during the trial you should contact the trial doctor immediately and stop taking your trial medication. You should discuss your participation in this trial with any insurance provider you have (e.g. protection insurance, life insurance, income protection, critical illness cover and private medical insurance) and seek advice if necessary, as failure to notify them may affect or invalidate your cover.