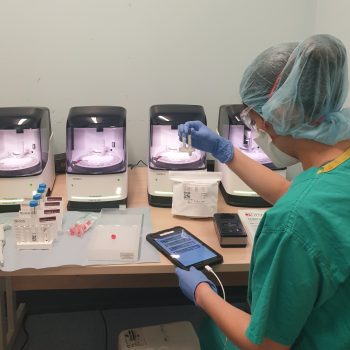
Speeding up the diagnosis of COVID-19 in a hospital setting using a SAMBA II
The first part of the study will evaluate the accuracy of the new SAMBA II-based test and whether it speeds up the diagnosis of COVID-19 in a ‘real-time’ hospital setting at the point of care.
The second part will investigate a point of care finger prick ‘antibody’ test of the blood, to determine how quickly markers of immunity appear following infection and a positive SAMBA test. These antibody tests will be important for understanding which patients and staff have already had the infection, and may be safe to return to work following recovery.
Research nurses from the NIHR Cambridge CRF are collecting samples from patients with suspected COVID-19 to support the COVIDx study, using the SAMBA II machine to test nasal and throat swabs to determine if a patient has COVID-19 and if the new device is an improved source of testing.
The SAMBA II test can provide extremely reliable results in less than two hours, meaning decisions about clinical care or self-isolation can be made much more rapidly. The antibody tests require serum from blood samples, which will be tested in specialised facilities at the Cambridge Institute for Therapeutic Immunology and Infectious Diseases (CITIID).
Once the two diagnostic tests have been validated in patients with confirmed COVID-19, the study will enrol a second group of participants – healthcare workers. The SAMBA II test will be able to quickly identify staff who are positive for COVID-19, even if they have no symptoms, allowing them to self-isolate or access treatment if required.
This is an abridged version of the news item posted on our website on April 17, 2020.