Rapid COVID-19 test halves patient waiting times
A new diagnostic test that can diagnose COVID-19 in a hospital setting faster than standard testing methods has been able to halve patients waiting times.
Originally used for diagnosing HIV across Africa, SAMBA II machines were deployed in the ED and holding wards at Addenbrooke’s hospital to help detect COVID-19 in patients as part of a research trial called COVIDx.
Researchers investigated whether using the new machines could accurately provide a faster diagnosis than standard testing practices and review how it would affect patient waiting times.
After collecting nose and throat swabs from over 140 patients, the samples were processed using the SAMBA II machine. Researchers found they were able to provide an accurate diagnostic result within 90 minutes, compared to the standard 24-48-hour lab waiting time.
Researchers also reviewed the length of time patients spent in on the ‘holding’ ward before they were admitted to the hospital or discharged. They compared the electronic patents records of all those who had in-hospital tests done in the 10 days before and then after the switch to diagnosis by SAMBA II machines at the hospital.
They found waiting times halved from 58.5 hours to 30 hours and saw a fall in the use of single-occupancy ‘isolation’ rooms in which COVID-19 patients are ideally treated, freeing up hospital beds.
With this new evidence, the hospital has switched most of their testing methods to the SAMBA II machines to diagnose patients with suspected COVID-19.
Professor Ravi Gupta from the Cambridge Institute for Therapeutic Immunology and Infectious Disease, who led the COVIDx study said: “Rapidly testing admissions for SARS-CoV-2 at the point of care is essential for reducing COVID-19 transmission in hospitals, speeding up access to urgent care and allowing safe discharge to care homes. Keeping surgical bays open means fewer cancelled operations and speeding up access to life-saving clinical intervention.”
Researcher, Dr Dami Collier said: “Our research demonstrates that point-of-care testing with SAMBA II machines is not only reliable, accurate and much faster, but that the diagnostic speed leads to significant real-world improvements for patient care and safety.”
CUH Medical Director, Dr Ashley Shaw, said: “Point of Care testing has been hugely beneficial in enabling our clinical teams to make well-informed and timely decisions, keeping patients and staff as safe as possible throughout this difficult period.”
The COVIDx study was supported by the NIHR Cambridge Biomedical Research Centre and NIHR Cambridge Clinical Research Facility.
Cambridgeshire Trusts join COVID-19 vaccine trial
Three leading NHS Trusts in Cambridgeshire are collaborating on a COVID-19 vaccine trial which has been developed by the University of Oxford.
The COV002 trial aims to assess how well people across a broad range of ages could be protected from COVID-19 using a new vaccine called ChAdOx1 nCoV-19. It will also provide valuable information on safety of the vaccine and its ability to generate good immune responses against the virus.
Cambridgeshire and Peterborough NHS Foundation Trust (CPFT), Cambridge University Hospitals NHS Foundation Trust and Royal Papworth Hospital NHS Foundation Trust are offering an opportunity for healthy staff who aged between 18-55 years old, who have not been infected with coronavirus but have regular face to face contact with COVID-19 patients, to take part in the trial. CPFT is the only integrated physical, mental health and social care NHS Trust in the UK offering the trial to staff across the region.
If they are eligible to take part in the study, participants will be randomised to receive one dose of either the trial vaccine (ChAdOx1 nCoV-19) or a licensed meningitis vaccine (MenACWY) that will be used as a ‘control’ for comparison. Screening of participants began on 29 May 2020 and following vaccination, participants will be followed up over 12 months.
Dr Estée Török, Honorary Consultant in Infectious Diseases and Microbiology and Principal Investigator at Cambridge University Hospitals NHS Foundation Trust, said: “Developing an effective vaccine is key to controlling the COVID-19 pandemic. We are delighted to be working with CPFT and RPH on this UK national priority vaccine trial. We are looking for healthy volunteers at high risk of COVID-19 infection at CUH to participate in this study and are most grateful to them for doing so.”
Dr Ben Underwood, Deputy Medical Director and Principal Investigator (study lead) at CPFT said: “We are grateful to all our staff for their brilliant response to the coronavirus pandemic. Our research teams are playing a vital role in international efforts to secure a vaccine, which we hope will protect those most at risk, save more lives and minimise the disruption caused by the virus. Thank you to all volunteers who take part and make clinical trials possible.”
Dr Robert Rintoul, Director, Papworth Trials Unit Collaboration, said: “We at Royal Papworth Hospital are proud to be supporting research into possible vaccines and treatments for COVID-19. I would like to thank our staff members who have chosen to participate in this important public health project.”
This study is funded and supported by the National Institute for Health Research and UK Research Innovation.
For further information on the trial visit the University of Oxford website.
Cambridge study to trial widely used drug to protect healthcare staff from COVID-19.
The PROLIFIC study (ChemoPROphyLaxIs For covId-19 infeCtious disease) aims to determine  whether hydroxychloroquine (HCQ), a widely used anti-malarial drug, is effective at preventing or reducing COVID-19 infection in frontline healthcare workers.
whether hydroxychloroquine (HCQ), a widely used anti-malarial drug, is effective at preventing or reducing COVID-19 infection in frontline healthcare workers.
Until a safe and effective vaccine is available to prevent SARS-CoV-2 infection and the development of COVID-19 symptoms, NHS and other frontline healthcare staff, including paramedics, will continue to remain vulnerable even with the best personal protective equipment (PPE) available. Chemoprophylaxis (drugs taken to prevent infection) may be an effective strategy for reducing infection in NHS staff until a successful vaccine can be produced.
Although HCQ and the related drug, chloroquine, have been suggested as potentially effective treatments for COVID-19 from early on in the pandemic, there have not yet been any well-designed, sufficiently large studies that can provide the evidence required for their use as preventative therapies. PROLIFIC is a randomised, double-blinded and placebo-controlled study that will determine whether daily or weekly doses of HCQ are effective at reducing COVID-19 infection among front-line health care workers.
HCQ, a medication already widely and safely used to prevent malaria in high risk countries has shown promise at preventing SARS-CoV-2 infection in vitro (in cells grown in a lab). Compared with chloroquine, HCQ causes fewer side effects, and has shown greater potential at preventing coronavirus infection in early studies, even when used at lower concentrations. How HCQ works to prevent infection is not fully understood, but researchers believe that it may block the virus from entering cells by influencing the pH in the part of the cell that would normally fuse with the virus to allow it to get inside the cell and replicate.
Dr Joseph Cheriyan, Consultant in Clinical Pharmacology at Cambridge University Hospitals, and Chief Investigator of the study said: “It is important that we quickly determine via a randomised controlled trial if HCQ provides a means to provide additional protection to frontline workers, this in turn will also protect the public. The PROLIFIC trial should provide answers shortly to address these issues.”
If this trial is successful, it will help to protect and maintain our skilled healthcare workforce, as well as reducing the burden of infection on an already strained healthcare system.
Professor Ian Wilkinson (Professor of Therapeutics and Director of the Cambridge Clinical Trials Unit) said: “Front-line heath care workers are like the spitfire pilots of the Battle of Britain. I am delighted that Cambridge is leading the way in testing hydroxychloroquine to protect health care workers from a disease that has already claimed the lives of too many people. PROLIFIC will be based exclusively in the UK and thus provide an answer directly relevant to the NHS and UK social care setting.”
PROLIFIC will be recruiting healthcare participants from hospitals and care homes across the country.
Find out more about the PROLIFIC trial and read the patient information sheet, or for further information visit the CCTU website.

Over 150 participants recruited to new COVID-19 research programme
A “library” of valuable biological samples from people with COVID-19, which will help scientists globally fight the virus, has recruited its 150th participant.
The National Institute for Health Research (NIHR) BioResource began collecting samples at the end of March from patients admitted to Addenbrooke’s hospital with suspected COVID-19. Samples included blood, nasal and throat swabs, plus a mental and physical health questionnaire.
The biological samples are carefully separated, analysed and stored as part of a resource for scientists to draw upon when researching treatments, vaccinations or a deeper understanding of COVID-19.
The NIHR BioResource will now seek further participants to join the new NIHR COVID-19 BioResource cohort. All hospital patients in Addenbrooke’s and The Royal Papworth Hospital with suspected or confirmed COVID-19 are invited to take part in this essential research. NHS staff undergoing routine screening for the COVID-19 infection will also be invited to participate in the study.
Up until now, local samples have mainly been collected in Cambridge, and processed in a specialist lab in the Jeffrey Cheah Biomedical Centre. Over the next few weeks, local NIHR BioResource centres across England will be able to begin recruiting from other hospitals.
Sample collection
Since the initiative launched in March, the NIHR COVID-19 BioResource has collected samples from more than 150 participants, including patients with COVID-19 and NHS staff who are either asymptomatic or with mild symptoms who had been self-isolating at home as a precaution. Collection of samples from NHS staff in addition to patients allows the NIHR COVID-19 BioResource to safely collect samples from participants with the full range of symptoms experienced from COVID-19, while contributing to the essential COVID-19 screening of NHS staff.
Dr Nathalie Kingston, Director of the NIHR BioResource said: “Since the outbreak, leading experts across the world are coming together to collaborate in joint research projects to help slow down and halt the disease, the NIHR BioResource is no exception.
“Working with our current infrastructure, we have created a new resource where we can easily recruit patients who have been diagnosed with COVID-19 and clinical staff working in the NHS. We can then track the disease progression during the participant’s recovery as well as understand any changes to their mental health. We will then be able to harness and use the information for future research trials and improve our knowledge of the disease.
“We have a special interest in enrolling patients under 40 who are admitted to hospital, to better understand their immune response. We will also be working alongside paediatrics intensive care units across the country to enrol patients under the age of 16 and their parents.
“The NIHR COVID-19 BioResource doesn’t prevent anyone from taking part in other research studies for COVID-19. There are many national research studies taking place and we need as much information as possible if we are going to tackle this disease.”
How the NIHR BioResource helps research
The NIHR BioResource is a nationwide platform that supports research in a number of health conditions. It brings patients, as well as healthy volunteers, who want to take part in research together with researchers who need volunteers to take part in studies. Currently, there are over 150,000 people with or without conditions who have consented to be approached to participate in research.
The NIHR COVID-19 BioResource offers patients diagnosed with COVID-19 and clinical staff an opportunity to provide their biological samples for the purposes of research. Healthy volunteers are currently able to sign up without providing a sample but can consent to take part and will complete a health questionnaire. They will then be invited to provide their sample at a later date when restrictions are lifted and when it is safe to do so.
Researchers will use the information to support current and future research into understanding the disease, including finding new treatments and why some people experience mild symptoms but others are more serious.
Principle Investigator Professor Ken Smith from the Cambridge Institute of Therapeutic Immunology and Infectious Disease will be analysing the samples collected from Cambridge to understand how the immune responds to the virus.
Professor Smith said: “By understanding more about participants’ immune response to the virus, we hope to find out why some people get severe, and indeed life-threatening, COVID-19 while others have very mild symptoms or no symptoms at all.
“This information will help in the development of effective vaccines and may help doctors to identify people who are more at risk of severe symptoms before they are infected.”
Dr Michael Weekes, Honorary Consultant in infectious diseases, whose primary research focuses on how our cells defend themselves from viruses, is trying to identify people most at risk of severe COVID-19. Dr Weekes said: “We have been studying blood samples from participants of the NIHR COVID-19 BioResource for use with a technique called ‘proteomics’.
“This is where we analyse which proteins are present on the surface of white blood cells from patients with severe or milder forms of COVID-19. This will help us determine when a patient is admitted to hospital with COVID-19 if it is likely they will need to be treated in the intensive care unit.
“We are also looking at healthcare workers who have experienced mild symptoms of COVID-19, or even who have had the virus without any symptoms at all. Staff who have signed up to the NIHR COVID-19 BioResource have provided a small sample of blood to be analysed. This research will help us understand how our body defends itself against the virus, and identify who is most at risk of developing severe COVID-19.”
Take part without being an inpatient
People who have had mild COVID-19 symptoms or have not been affected with the virus can still get involved in research. Currently, there is no way of safely collecting biological samples from a non-hospital setting. However, people can still contact the NIHR BioResource to volunteer for research in other ways, such as taking surveys.
The NIHR COVID-19 BioResource is encouraging more participants who have been hospitalised with COVID-19 to sign up to the research programme. The more samples collected, the more chance researchers can understand the disease and discover new treatments. www.bioresource.nihr.ac.uk
Genetic analysis reveals new causes of Primary Immunodeficiency (PID)
A UK-wide research collaboration through the National Institute for Health Research (NIHR) BioResource for Rare Diseases has combined whole genome sequencing (WGS) with a statistical program called BeviMed to predict the existence of disease-causing changes in the DNA of people with Primary Immunodeficiency (PID).
PID is a severe condition with a range of symptoms, including repeated severe (and often life-threatening) infections, autoimmunity (where the body’s immune system attacks itself) and early onset of cancers. PID is challenging to diagnose and treat, but understanding the genetic cause of the condition in each individual can help to identify the most effective treatment.
In this NIHR Cambridge Biomedical Research Centre (BRC) funded study published in Nature (06 May), the research team sequenced the entire genetic code of 974 people with PID. The team were able to identify variations (changes) in genes already known to cause PID in almost 1 in 5 of the study participants, providing them with a genetic diagnosis for their condition.
To help identify genetic causes for the remaining participants and other patients with PID, the team used a statistical program known as BeviMed. BeviMed can be used to predict genes that may cause PID, by comparing the genomes of cases (people with PID) and controls (people without the condition). Using this technique, the team were able to identify new genes that cause PID.
The paper’s lead author, Dr James Thaventhiran, said: “The use of whole genome sequencing allows us to rapidly identify known genetic causes of immunodeficiency. The scientific advance comes from using sophisticated analytical methods to discover new genetic causes of PID. These results are important for our patients because identification of the genetic cause may allow us to prioritise the therapeutic targeting of specific immune pathways to improve their health”.
The study also provided a new insight into how multiple genetic defects can act together to cause diseases such as PID. The research team were able to identify families of patients where different combinations of common and rare genetic changes gave rise to different forms of PID, with varying symptoms and severity.
This research may also help to explain why some patients with the same form of PID experience vastly different symptoms. Professor Adrian Thrasher, a lead investigator and Consultant in Immunology based at Great Ormond Street Hospital (GOSH) explained: “Until now, the reasons for this variability have been very unclear. WGS has allowed us to map out unique combinations of genetic errors in each patient and consider how their interactions could lead to different symptoms.”
Combined analysis approaches to whole genome sequences from large numbers of patients with a rare disease type, such as PID, can provide new understanding of the genetic causes of these diseases. This is important in PID because it will improve diagnosis rates and allow more targeted treatment and advice, ultimately improving outcomes for patients.
Dr Susan Walsh, Director of the patient support group, Primary Immunodeficiency UK, said: “Knowledge is power, and we hope techniques like these become routine for future patient diagnosis and treatment. These findings are providing some PID patients with answers, ending their diagnostic odyssey, and helping define what the best treatments are for these individual patients. We look forward to hearing about and collaborating on further studies and the opportunity for more patients to take part in this important research.”
Advances in the understanding of rare disease are only made possible through programmes such as the NIHR BioResource Primary Immunodeficiency Collaboration, that brings together large numbers of people affected by the disease who are willing to take part in research with leading experts in rare diseases and immunology from across the country and the world.

Professor Ken Smith
“By bringing together expertise in these rare diseases from across the UK and recruiting a larger number of individuals affected by them, we hope to be able to provide more genetic diagnoses and better treatments for patients,” said Chief Investigator and Director of the Cambridge Institute of Therapeutic Immunology & Infectious Disease (CITIID), Professor Ken Smith. “At the same time, by understanding what goes wrong in the immune systems in rare cases, we hope to provide fresh insights into how the human immune system works more generally, which could ultimately benefit a much wider number of patients.”
The team have now been awarded a £4 million Collaboration Award from the Wellcome Trust to take the research forward. The new collaboration includes the NIHR BioResource and Genomics England, which, together with NIHR Biomedical Research Centres in England, will support the UK-wide consortium to expand the number of PID patients undergoing both WGS and detailed immune investigations, to further advance our ability to diagnose PID.
Professor Smith continued: “This approach allows gene discovery in adult patients without known affected family members and with complex disease – a group in which this has previously been very difficult. Its application to other rare adult diseases therefore has the potential to allow similar advances. It will be applied, for example, to a cohort-based whole genome sequencing study being planned to investigate the genetic drivers of susceptibility to severe COVID-19”.
NIHR Cambridge BRC and CRF begin working on new COVID-19 study
Research staff from NIHR Cambridge Clinical Research Facility (CRF) are being deployed onto the wards at Cambridge University Hospitals (CUH) to start using a new test for a research trial into COVID-19.
COVIDx, a study supported by the NIHR Cambridge BRC, aims to investigate the impact of two new tests for COVID-19 on delivering faster diagnoses and understanding the development of immunity following infection. The first part of the study will evaluate the accuracy of the new SAMBA II-based test and whether it speeds up the diagnosis of COVID-19 in a ‘real-time’ hospital setting at the point of care.
The second part will investigate a point of care finger prick ‘antibody’ test of the blood, to determine how quickly markers of immunity appear following infection and a positive SAMBA test. These antibody tests will be important for understanding which patients and staff have already had the infection, and may be safe to return to work following recovery.
Research nurses detecting COVID-19
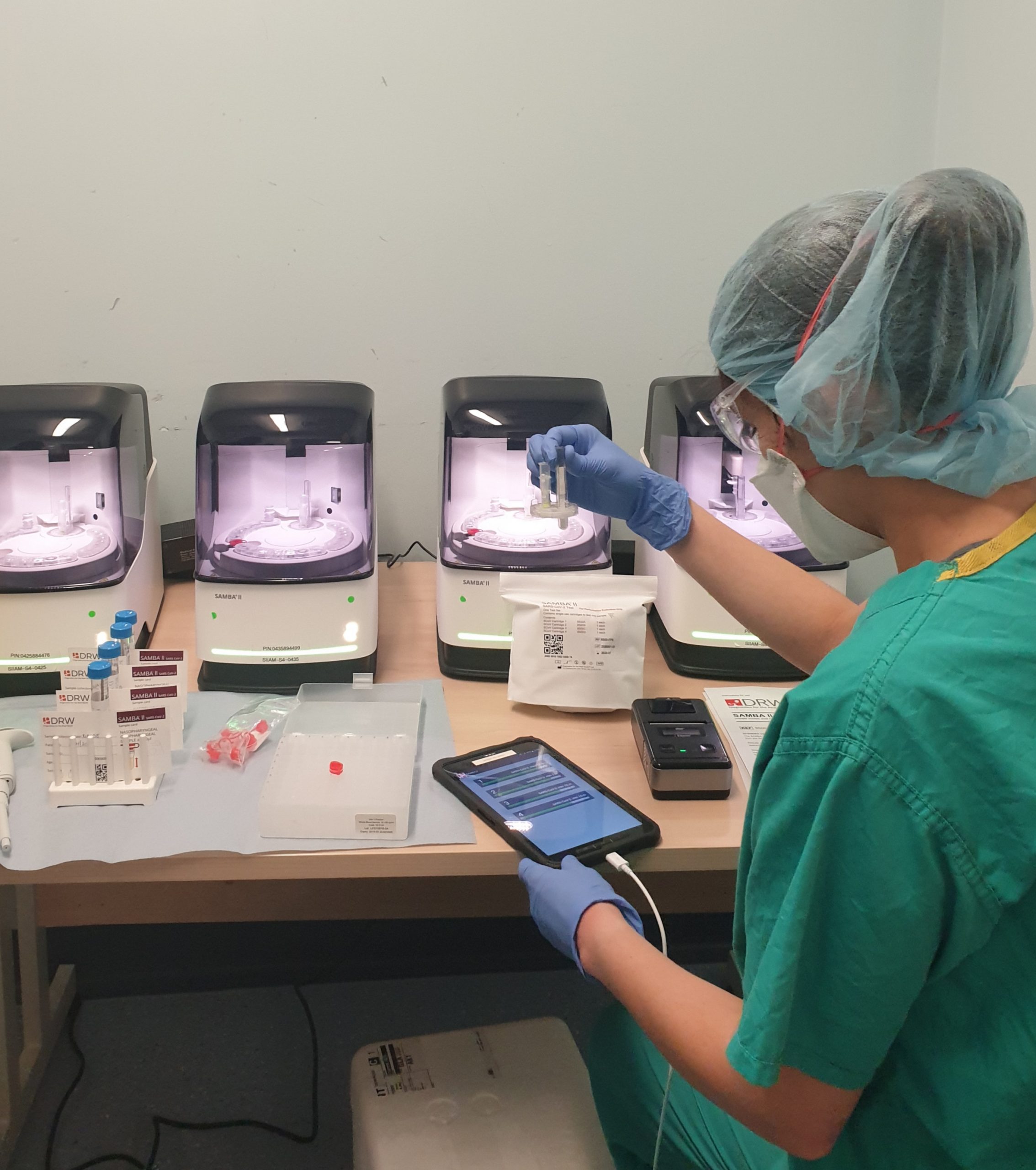
Research nurses working with the SAMBA II machines
Research nurses from the NIHR Cambridge CRF are collecting samples from patients with suspected COVID-19 to support the COVIDx study. Vivien Mendoza, NIHR Cambridge CRF lead research nurse said: “Our research nurses are working on a triage ward at CUH dedicated for patients who may have COVID-19.
“We are using the SAMBA II machine to test nasal and throat swabs to determine if a patient has COVID-19 and if the new device is an improved source of testing. The new test only takes 90 minutes and we will be matching these swabs against the results of standard practices.”
Currently, swabs taken from patients are sent off to the lab to be analysed, which can take a few days to return a result. The SAMBA II test can provide extremely reliable results in less than two hours, meaning decisions about clinical care or self-isolation can be made much more rapidly. The antibody tests require serum from blood samples, which will be tested in specialised facilities at the Cambridge Institute for Therapeutic Immunology and Infectious Diseases (CITIID).
Vivien added: “SAMBA II produces a summary of the test and the results are sent to a tablet and can be forwarded on to the clinician. When the clinicians receive the results they are able to start the correct care pathway for the patient a lot faster. We will also be testing NHS staff members who are working in COVID-19 wards and ITUs.”
The new test will help those who may have been infected and those who are displaying non-specific symptoms (such as from hay fever or the common cold) return to work. This will avoid healthy staff going into self-isolation, supporting the delivery of front-line care.
Caroline Saunders, Director of Clinical Operations for NIHR Cambridge CRF said: “Research is vital in finding new treatments and testing new devices, COVID-19 shows exactly what our staff are trained for. Our nurses have tremendous experience and expertise in trialling new devices and treatments across a wide range of specialties and age ranges and have the skills to adapt to these new challenges.”
Testing patients and staff
Patients who are admitted to Cambridge University Hospitals and have suspected COVID-19 will be given the opportunity to take part in the COVIDx research trial, providing swabs and blood samples. They will also be given the opportunity to enrol in other COVID-19 related trials such as the RECOVERY study, currently on going at the hospital.
Once the two diagnostic tests have been validated in patients with confirmed COVID-19, the study will enrol a second group of participants – healthcare workers. The SAMBA II test will be able to quickly identify staff who are positive for COVID-19, even if they have no symptoms, allowing them to self-isolate or access treatment if required. The antibody test will be able to identify staff who have already had the infection, which will support frontline NHS staff over the epidemic.
Professor Ravi Gupta from the Cambridge Institute for Therapeutic Immunology and Infectious Disease, who is leading the COVIDx study said: “Like all new medicines, new tests and devices need to be assessed for their strengths and weaknesses. COVIDx is a month-long trial at CUH which will give us an opportunity to evaluate two new forms of point of care testing for COVID-19 in ‘real life’.
“By comparing 200 patient samples to the new screening tool, we will be looking at whether the new tests are faster and are more reliable than the existing practices. If we can prove the new test can provide a diagnosis in a fraction of the current waiting time, then we are able to triage patients to the right part of the hospital earlier and start their care a lot sooner.
“We are beginning to test staff in high-risk areas to help reduce the risk of infection and stop staff having to go into self-isolation unnecessarily. It is really important we can ramp up tests for everyone to make sure we can minimise the risk of infection and have better patient outcomes.”
Further information on SAMBA II
The technology behind SAMBA II was developed while Dr Helen Lee was at Cambridge’s Department of Haematology. The development of the technology has been supported by Wellcome, the Children’s Investment Fund Foundation, the US National Institutes of Health and Cambridge Enterprise, among others. For more information see the University of Cambridge press release
The SAMBA II has been initially rolled out at CUH before being launched in hospitals nationwide to test patients, and staff working in high-risk areas or intensive care units.
World’s first artificial pancreas app licensed for people with type 1 diabetes in UK
Pioneering diabetes researcher Professor Roman Hovorka launched the world’s first licensed, downloadable artificial pancreas app for people with type 1 diabetes.
The commercial launch is a milestone in the journey towards full artificial pancreas technology for everyone with type 1 diabetes.

Professor Roman Hovorka
The CamAPS FX app works with an insulin pump and a glucose monitor to automatically deliver insulin to people with this life-threatening condition via a complex algorithm.
Currently, people with type 1 diabetes rely on a routine of finger-prick blood tests and insulin injections or infusions just to stay alive, because their pancreas no longer produces insulin itself.
The app – which Professor Hovorka hopes will become available in the future on the NHS – will take over much of the management of the condition. This is particularly important at night, when many people with type 1 diabetes experience potentially dangerous low blood glucose levels.
The app can also upload the user’s blood glucose measurements seamlessly to Diasend, an online platform, allowing their diabetes team to provide more personalised care.
The CamAPS FX app is backed by 13 years of clinical research carried out by Professor Hovorka and his research group, and funded by the type 1 diabetes charity JDRF, Diabetes UK, the National Institute for Health Research Cambridge Biomedical Research Centre, the National Institutes of Health, Horizon 2020, and The Leona M and Harry B Helmsley Charitable Trust.
It is licensed for use by both adults and children with the condition – including pregnant women, and children aged one and up.
It is the first artificial pancreas system to be licensed for use in pregnancy, or by young children.
The app is now available for UK users to download onto Android phones via the Amazon Appstore.
The app is available on a subscription basis starting at £70 per month.
At launch, the app will be supported by a small number of UK diabetes clinics. People who wish to use the app will need to confirm which clinic they attend, and must be using a Dana RS pump and a Dexcom G6 continuous glucose monitor.
But Professor Hovorka and his research team will work to continue to bring this technology to all who need it, via the NHS. Key to this will be the generation of data to support the case for NHS provision.
The CamAPS FX app is a first step towards a fully automated and interoperable artificial pancreas.
Fully automated and interoperable artificial pancreas technology will fundamentally change life with type 1 diabetes, by working with a range of insulin pumps and glucose monitors to lift the burden of managing a condition that is relentlessly unpredictable day and night.
Professor Hovorka will continue refining the artificial pancreas through research into mealtime glucose control and improving ease of use.
Professor Hovorka, Professor of Metabolic Technology at the University of Cambridge, said: “This is a major stepping stone towards providing widely available, clinically proven, and user friendly artificial pancreas technology to people with type 1 diabetes.
“Our aim is to alleviate the ever-present burden of type 1 diabetes and improve health outcomes. This is the outcome of hard work, with more to come. We are indebted to all who are helping us on this journey.”
Karen Addington, UK Chief Executive of type 1 diabetes research charity JDRF, said: “JDRF is proud to have supported Professor Hovorka’s artificial pancreas research from the beginning, nearly 15 years ago. This app is a major innovation and a significant milestone on the road to a fully automated and interoperable artificial pancreas. “There’s still more work to do, but this is an exciting step.”
Fiona O’Reilly, who has been using the app as part of a clinical trial, said: “Overall, it makes me feel free. It is the closest I have been to living without the burden of type 1 diabetes since I was diagnosed which is a fabulous feeling – I feel less fearful of hypoglycaemia, and less ashamed of the fact that I find achieving good glycaemic control so tricky.
“And it makes me feel more positive of my future with diabetes, that I have a chance of avoiding all the associated complications. It also makes me feel lucky to live in a time where this technology is possible and really grateful to be given the chance to try it out.”
Written by JDRF
Celebrating International Women’s Day
International Women’s Day is celebrated globally on the 08 of March every year. It’s focuses on highlighting women, calling for equal opportunities and removing discrimination. This years theme is ‘Each for Equal’.
The NIHR Cambridge BRC is focusing on some of the women who make substantial contributions to health research and why it is important that more women are needed in the science sector. Click on the picture below to reveal their journey into research.
“There are so many talented women, we need to support and push women forward and grow their confidence – women are just as capable, just as smart and can drive ideas to benefit the science community. My advice is you should seize every opportunity, do what makes you happy regardless of gender, stand up and say ‘yes, I can do this.’”
Dr Nathalie Kingston, Director of NIHR BioResource for Translational Research
“I see a lot more women involved in research, whether that is leading their own research studies, running labs or coordinating research and it’s fantastic. I work with some wonderful women in senior positions and they are great examples of women working at the top and making a difference.”
Jo Piper, NIHR Cambridge Clinical Research Facility Manager
“Women can bring different perspectives, questions and ways of working that can enrich the science sector. It’s important that women globally are not limited by the types of careers that are open to them.”
Professor Christi Deaton, Florence Nightingale Foundation Clinical Professor of Nursing at the University of Cambridge and Cambridge University Hospitals
“In the last five years there has been a definite shift supported by senior management here to encourage women into more senior roles. We want our research environment to be merit-driven, judgement-free and open to everyone – whatever your age, gender or background – and we want to offer a career structure that offers flexibility and allows for family-raising responsibilities.”
Dr Jean Abraham, Director, Cambridge Breast Cancer Research Unit
“We need perspectives from all backgrounds, genders and ethnicities to ensure balanced research. We can offer mentoring or work experience and if your circumstances change, we advise everyone to keep one foot in the door, even if it’s just one day a week, because you never know the opportunities that will become available.
Seize every opportunity, just reach out and grab it! If you’re ambitious and willing to put the work in, you can achieve what you want – and you never know where it will lead.”
Professor Fiona Gilbert, Professor of radiology, NIHR Cambridge BRC Imaging theme lead
“I found research rewarding because that’s where we can provide the evidence to make the changes to how we treat people in the future. More women are needed in science and in medicine. The population we serve is diverse and therefore it is important that the workforce is equally diverse”
Sarah Hickman, PhD student, Clinical Research Associate
“I’ve always worked in teams filled with people from lots of different backgrounds with different perspectives and approaches, and that’s when the most creative ideas come together and the most interesting discoveries happen. Whatever the sector, it’s important that the best people are able to succeed regardless of their gender or background.
Dr Georgie Bowyer, Post-Doctoral Research Associate
Patients living with Crohn’s and colitis to benefit from £5m grant for a new data research hub based in Cambridge
- £5m grant awarded by Health Data Research UK (HDR UK) to establish a health data research hub for inflammatory bowel disease (IBD) following a successful bid led by Eastern AHSN and Cambridge University Health Partners (CUHP)
- The Cambridge based hub will be one of seven hubs set up across the UK to speed up research for new medicines and treatments, support quicker diagnoses and potentially save lives
- The IBD hub, to be known as G.I. Know, will work together with patients, industry, academia and the health service to transform our understanding of inflammatory bowel disease, drive improvements in diagnosis and treatment and deliver a data framework to reproduce in other disease areas
There is an urgent need to better understand why patients with Crohn’s disease and ulcerative colitis (collectively known as inflammatory bowel disease or IBD) respond differently to treatments so they can be prescribed the best personalised treatment as early as possible in order to improve outcomes, minimise surgery and reduce costs. Whilst advances in clinical imaging, pathology and particularly genomics have produced remarkable progress in understanding Crohn’s disease and ulcerative colitis the power of these technologies cannot be fully realised until their outputs are combined in a secure research resource and made accessible to the whole research community. This project makes that possible.
Mark Avery, Director of Health Informatics at CUHP and Eastern AHSN, who brought the bid team together commented; “This is such an exciting opportunity to be at the forefront of health data research. Working with the NIHR BioResource and patients we will transform our understanding of inflammatory bowel disease, drive improvements in diagnosis and treatment and deliver a data framework that could be used in future for other diseases. At Eastern AHSN we believe that citizens, academia, health services and industry will achieve more working together than they will in isolation. This project exemplifies that, and we are grateful to all our partners on this hub including the NIHR BioResource, Crohn’s and Colitis UK, the IBD Registry, Welcome Sanger, AIMES, Privitar and Microsoft.”
Crohn’s disease and ulcerative colitis are estimated to affect one in every 130 people in the UK (over 500,000) and costing UK health budgets approximately £1.5 Billion each year. Treatment is  with steroids, immunosuppressants and antibody therapies, but results are variable. Over 70% of patients with Crohn’s and 15% with colitis require major surgery. There is an urgent need to better understand why patients respond differently to treatments in order to improve outcomes and reduce costs.
with steroids, immunosuppressants and antibody therapies, but results are variable. Over 70% of patients with Crohn’s and 15% with colitis require major surgery. There is an urgent need to better understand why patients respond differently to treatments in order to improve outcomes and reduce costs.
Rosanna, Crohn’s disease patient commented; “Patients with inflammatory bowel disease need to find the most effective treatment as quickly as possible to limit disease progression – but currently this process can take three or more years of trial and error. This initiative makes the tantalising prospect of personalised medicine real for patients, who for the first time will have the confidence that they have been prescribed the most effective treatment for them from the start.”
Patient and public involvement is key to the success of this bid. 25,000 IBD patients across 90+ NHS Trusts have already been recruited to an NIHR BioResource and provided consent for their health records to be used for medical research.
A life of low cholesterol and BP slashes heart and circulatory disease risk
Countless heart attacks and strokes could be prevented with sustained drop in cholesterol and blood pressure
Modest and sustained decreases in blood pressure and cholesterol levels reduces the lifetime risk of developing fatal heart and circulatory diseases, such as heart attack and stroke, according to research. The findings are being presented at the European Society of Cardiology (ESC) Congress in Paris and published in the Journal of the American Medical Association (JAMA).
Researchers have found that a long-term reduction of 1 mmol/L low-density lipoprotein (LDL), or ‘bad’ cholesterol, in the blood with a 10 mmHg reduction in blood pressure led to an 80 per cent lower lifetime risk of developing heart and circulatory disease. This combination also reduced the risk of death from these conditions by 67 per cent.
The team found that even small reductions can provide health benefits. A decrease of 0.3 mmol/L LDL cholesterol in the blood and 3 mmHg lower blood pressure was associated with a 50 per cent lower lifetime risk of heart and circulatory disease.
Scientists have previously found that lowering both blood pressure and the amount of ‘bad’ cholesterol in the blood are two ways which can prevent the onset of heart and circulatory disease. However, the risk, which accumulates over time, has not been quantified before.
In this study, Professor Brian Ference and his team studied 438,952 participants in the UK Biobank, who had a total of 24,980 major coronary events – defined as the first occurrence of non-fatal heart attack, ischaemic stroke or death due to coronary heart disease. They used an approach called Mendelian randomisation, which uses naturally occurring genetic differences to randomly divide the participants into groups, mimicking the effects of running a clinical trial.
People with genes associated with lower blood pressure, lower LDL cholesterol and a combination of both were put into different groups, and compared against those without these genetic associations. Differences in blood LDL cholesterol and systolic blood pressure (the highest level that blood pressure reaches when the heart contracts), along with the number of cardiovascular events was compared between groups.
Professor Brian Ference now hopes that these findings can bring about change in the healthcare of people at greater risk of developing heart and circulation complications, and improved guidance for those requiring lifestyle changes.
Professor Brian A Ference, lead researcher of the study at University of Cambridge, said:
“Heart and circulatory diseases steal the lives of 168,000 people each year in the UK, which is just greater than the population of the city of Cambridge. It’s vital we do everything possible to help prevent people developing these life-threating conditions.
“Even small reductions in both ‘bad’ cholesterol and blood pressure for sustained periods of time can pay very big health dividends, and dramatically reduce the lifetime risk of developing heart and circulatory disease.”
“We now plan to take the results from this study to create a lifetime cardiovascular risk calculator and to support the development of new prevention guidelines.”
Professor Sir Nilesh Samani, Medical Director of the British Heart Foundation said:
“This research again demonstrates that high blood pressure and raised cholesterol are key risk factors for heart attacks and strokes. But how many of us know our numbers for these, or have made sustained efforts to lower them? Hopefully, the findings reported shows that the risk could be reduced by as much as 80 per cent, can act as a motivator for long-term change.
“Millions of people are living with untreated high blood pressure or raised cholesterol, both of which can be lowered with lifestyle changes and medication. Huge numbers of heart attacks and strokes can be prevented simply by getting to know your numbers and taking your health into your own hands.
“Simple devices are now available for measuring blood pressure. Also, everyone between the ages of 40-74 is eligible for a free NHS health check, which assesses your risk of developing heart and circulatory diseases, and includes cholesterol and a blood pressure reading. It’s important that we all take advantage of this.”
This research was funded by the BHF, UK Medical Research Council (MRC) and the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre.
Written by BHF
New stem cell combination could help to repair damaged hearts
A combination of heart cells derived from human stem cells could be the answer to developing a desperately-needed treatment for heart failure, according to new research part-funded by the British Heart Foundation (BHF), NIHR and supported by the NIHR Cambridge BRC and published in Nature Biotechnology.
Researchers have found that, by transplanting an area of damaged tissue with a combination of both heart muscle cells and supportive cells taken from the outer layer of the heart wall, they may be able to help the organs recover from the damage caused by a heart attack.
Scientists have been trying to use stem cells to repair damaged hearts for a number of years. Efforts have been unsuccessful so far, mainly because the vast majority of transplanted cells die within a few days.
Now, Dr Sanjay Sinha and his team at the University of Cambridge, in collaboration with researchers at the University of Washington, have used supportive epicardial cells developed from human stem cells to help transplanted heart cells live longer.
The researchers used 3D human heart tissue grown in the lab from human stem cells to test the cell combination, finding that the supportive epicardial cells helped heart muscle cells to grow and mature. They also improved the heart muscle cell’s ability to contract and relax (1).
In rats with damaged hearts, the combination also allowed the transplanted cells to survive and restore lost heart muscle and blood vessel cells.
Researchers now hope to understand how the supportive epicardial cells help to drive heart regeneration. Understanding these key details will bring them one step closer to testing heart regenerative therapies in clinical trials.
Hundreds of thousands of people in the UK are living with debilitating heart failure, often as a result of a heart attack. During a heart attack, part of the heart is deprived of oxygen leading to death of heart muscle. This permanent loss of heart muscle as well as subsequent scarring combines to reduce the heart’s ability to pump blood around the body.
People suffering from heart failure can’t regenerate their damaged hearts and the only cure is a heart transplant. Ultimately, these researchers hope that, by harnessing the regenerative power of stem cells, they will one day be able to heal human hearts using a patient’s own cells.
In addition to the BHF, this research was funded by the UK Medical Research Council (MRC) and the National Institute for Health Research (NIHR).
Dr Sanjay Sinha, BHF-funded researcher and leader of the study at the University of Cambridge, said: “There are hundreds of thousands of people in the UK living with heart failure – many are in a race against time for a life-saving heart transplant. But with only around 200 heart transplants performed each year in the UK, it’s absolutely essential that we start finding alternative treatments.
Dr Johannes Bargehr, first author of the study at the University of Cambridge said: “Our research shows the huge potential of stem cells for one day becoming the first therapy for heart failure. Although we still have some way to go, we believe we’re one giant step closer, and that’s incredibly exciting.”
Professor Sir Nilesh Samani, Medical Director at the British Heart Foundation which part-funded the research said: “Despite advances in medical treatments, survival rates for heart failure remain poor and life expectancy is worse than for many cancers. Breakthroughs are desperately needed to ease the devastation caused by this dreadful condition.
“When it comes to mending broken hearts, stem cells haven’t yet really lived up to their early promise. We hope that this latest research represents the turning of the tide in the use of these remarkable cells.”
Cambridge University Hospitals revealed as country’s top performing trust for increasing research
Cambridge University Hospitals NHS Foundation Trust (CUH) has been revealed as England’s top performing trust for increasing research in the annual National Institute for Health Research (NIHR) Research Activity League Table, published today.
Research teams at the Trust ran 17% more studies compared to the previous year with 466 in total. This increase of 68 studies from 2018/19 is the highest of any Trust in England.
The Trust has now moved into the top 10 nationally for the overall numbers of patients taking part in health research. With 10,923 patients participating over the past year CUH is 7th for overall number in the country, up from 11th the previous year. This was a 23% increase from 2017/18 when 8,892 patients took part.
The Research Activity League Table is published by the NIHR annually to detail research activity across all NHS trusts and Clinical Commissioning Groups (CCGs) in England. The table provides a picture of how much clinical research is happening in which NHS organisations, and involving how many patients.
More people than ever before took part in NHS research in the Eastern region over the last year, according to the table. NHS and Social Care research teams from across the Eastern region ran over 963 NIHR studies, with 47,599 patients and public taking part in 2018/19 which is over 5000 more volunteers than last year (5,574). NHS trusts in the Eastern region saw an 80% increase research activity in comparison to 65% of NHS trusts in England.

Prof John Bradley
Prof John Bradley, Director of Research at CUH said: “We are delighted to be the top performing Trust for increasing our research activity, and to see ourselves in the top 10 for research participation. We strive to make sure research is accessible to everyone, and that participants have a good experience. Research is one of our core missions, and being highlighted in the league tables shows our dedication to delivering world-class research to improve healthcare.”
Dr Helen Macdonald, Chief Operating Officer for the NIHR’s Clinical Research Network in the Eastern region praised teams, saying: “It is fantastic to once again see the Eastern region playing such a strong national role in health and social care research. To have such a significant increase in the number of studies taking place shows that research in the region is thriving. It is also testament to the continued hard work and dedication of the research teams and health professionals who do so much to develop better treatments and care for patients now and in the future”.
Jonathan Sheffield, Chief Executive Officer of the NIHR Clinical Research Network says:
“The number of patients taking part in research this year is a significant step towards meeting the NHS Long Term Plan, of one million people being registered as interested in taking part in research by 2024. I would like to thank all those hard working NHS staff who are supporting the delivery of research in addition to their clinical duties, as we could not achieve these results without you.”
The table sits on the NIHR website at www.nihr.ac.uk/nihrleaguetable, accessible to anyone wanting to understand how much research activity is happening in their local trust or CCG.
To find out more about research taking place in the NHS and social care, visit the NIHR website www.nihr.ac.uk.

The Carr family
9 year old Mckenzie’s an NIHR research trailblazer!
Mckenzie Carr is the first participant to sign up to a new research study taking place at Cambridge University Hospitals NHS Foundation Trust (CUH) which looks at how sensitising the body to insulin can impact the effectiveness of growth hormone in short children who are born small. 9 year old Mckenzie, from Norwich, was considered much smaller than normal when he was born. Weighing just 4 pounds 7 ounces at birth, he had trouble feeding and failed to put on weight at the normal rate for newborns.
Parents, Carla-Jane and Kieran Carr, tried everything to get him to feed normally. However, unlike the majority of children who are born small, Mckenzie did not have a catch-up growth.
Mckenzie was just about to begin the standard course of growth hormones for short children who are born small, last year when mum, Carla-Jane, received a call from Julie a research nurse to tell her about the ‘SGA Metformin study’ being led by Professor David Dunger and Dr Ajay Thankamony at CUH. SGA is a term used for children who are Small for Gestational Age. The family were sent lots of information about the study which they considered and, once Mckenzie had given his approval, they agreed to join it.
Insulin, which the body naturally produces to help regulate blood sugar, has also been shown have an important role in the action of growth hormone. The study involves the participanttaking a daily dose of Metformin (a medication which makes the body more sensitive to insulin) or placebo (dummy drug) alongside their growth hormone injection which researchers hope will increase the effectiveness of the Growth Hormone. This takes place for 6 months, then the participant is monitored for another 6 months during which time they take the growth hormone on its own.
Mckenzie said, “I got a leaflet and read it and said yes because I wanted to know more about why I don’t grow”.
Dad, Kieran, has pointed out that the family decided to take part to help others as well, “As well as finding out why Mckenzie wasn’t growing, we hope taking part will help other people. It’s been a bit of a nightmare for him over the past 9 years so we hope that with this trial, someone else might be able to be helped in future.”
Professor Jeremy Turner, Consultant Endocrinologist and Clinical Lead for the NIHR’s Clinical Research Network which supports the study, said: “Research is a vital component in finding new treatments and it simply can’t happen without the involvement of patients like Mckenzie. We can’t thank him and all of our study participants enough for not only taking part, but also inspiring others to be part of research.”
To find out more about the SGA Metformin study email jh676@medschl.cam.ac.uk.
World-leading genome study spells hope for sick babies
A Cambridge-based study has shown that the diagnosis and treatment of some of the most critically ill babies can be improved by sequencing their whole genome.
The study, which is the largest of its kind in the world, uses advanced genome sequencing to help doctors identify genetic conditions in neonatal and paediatric intensive care units.
It comes as Cambridge prepares to build a new children’s hospital for the East of England, thanks to up to £100 million of Department of Health funding. Mapping the whole human genome and understanding the genetic basis of disease and recovery is central to the hospital’s vision.
The study found that one in four babies had an underlying genetic condition and that in the majority of the cases, the diagnosis changed their treatment plan. 
The Next Generation Children’s Project, uses whole genome sequencing that delivers results within a two to three week window in the National Health Service (NHS) in the UK. Proving that the tests are possible and have an impact on early diagnosis and treatment paves the way for this kind of testing to be offered nationally.
The breakthrough, which is expected to attract medical interest worldwide, will enable doctors to intervene earlier, manage conditions more effectively, potentially improve outcomes and even save lives.
Importantly, parents who lose a child are more likely to be spared the agony of not knowing the full reasons why, and will be able to make more informed decisions about trying for a family again.
The announcement follows a trial spearheaded by University of Cambridge and Cambridge University Hospitals NHS Foundation Trust (CUH), which runs Addenbrooke’s Hospital and The Rosie maternity.
With parents’ consent experts took blood samples from around 350 babies and children admitted to the neonatal and paediatric intensive care units with congenital abnormalities, neurological symptoms including seizures, metabolic disease, gastrointestinal disorders, or reduced growth. Parents also gave samples.
The study, funded by the National Institute of Health Research (NIHR), the Cambridge Biomedical Research Centre, the Rosetrees Trust and Isaac Newton Trust, discovered that one in four had  an underlying genetic condition. Critically, it changed the clinical management of 75 per cent of those patients.
an underlying genetic condition. Critically, it changed the clinical management of 75 per cent of those patients.
One of the surprising findings, which were scrutinised by top analysts, scientists, geneticists, critical care physicians and neurologists, was that a child’s appearance alone was rarely a good predictor of whether a child had a genetic condition and which gene abnormality caused the problems.
Chief Scientific Officer of NHS England, Professor Dame Sue Hill DBE, who is responsible for the development of genomics across the NHS said: “Genomics has the potential to transform the delivery of care for patients which is why the NHS has prioritised it in its Long Term Plan.
“This Cambridge trial is important because not only does it show the potential benefits of whole genome sequencing to significantly improve care for seriously ill children, but it also demonstrates this technology can be delivered as part of a mainstream NHS service. This trial will help to shape and inform the NHS implementation of whole genome sequencing for seriously ill children, as set out in the NHS Long Term Plan.”
University professor of pediatrics and CUH honorary neonatal consultant, David Rowitch, said: “I believe this study will have an enduring impact on the field. It adds to the weight of evidence that whole genome diagnostic testing should be applied in the management of intensively ill children.
“The promise of this for the future includes more rapid diagnosis and early detection of genetic disease that can pave the way to providing medicines that are best suited to the child.”
Professor of genomics and consultant clinical geneticist, Lucy Raymond, added: “We are proud that Cambridge is leading the nation on this cutting edge work with babies and young children and are really grateful to the families who have contributed to this study so generously.
“Each year almost 100,000 such patients are admitted to intensive care units across the UK and in the East of England alone it has the potential to help 3,000 annually.”
Cambridge researchers take top awards
Two NIHR Cambridge BRC researchers have won top awards at the The American Diabetes Association.
The American Diabetes Association’s (ADA) is a leading voluntary health organisation on a mission to prevent and cure diabetes, as well as improve the lives of all people affected by the disease.
Between 7-11 June, ADA held their 79th Scientific Session- the worlds largest scienetific meeting on diabetes research, prevention and care, in San Francisco. This was a chance to bring researchers across the world together to highlight diabetes research and recognise researchers in this field. Two Cambridge researchers were awarded at the conference for their significant discoveries.
Dr. Sadaf Farooqi was awarded the 2019 Outstanding Scientific Achievement Award for her work to understanding the genetics and physiological mechanisms in obesity, which will help develop new treatments to tackle this major health problem.
Also awarded, was our metabolism, endocrinology and bone theme lead, Prof. Sir Steve O’Rahilly. He was given the ADA’s highest honor, the Banting Medal for Scientific Achievement. The Banting Medal recognises significant, long-term contributions to the understanding, treatment or prevention of diabetes.
Study shows that in HER2 positive early breast cancer 6 months treatment with Herceptin is as good as 12 months for preventing cancer return
The majority of clinical trials in cancer assess either new treatments or additional treatments to the current standard of care. However equally important questions for patients and healthcare teams concern reduction in the length of treatments and attendant toxicities and whether this can be achieved without any worsening of outcomes. Clinical trial results published in the Lancet today show that women treated with 6 months of Herceptin for HER2 positive early breast cancer did as well in terms of their risk of breast cancer returning as those getting the currently conventional 12 months treatment. Six months also significantly reduced treatment related side effects, including heart problems.
The PERSEPHONE trial, a £2.6 million study funded by the NIHR Cambridge Biomedical Research Centre with translational research funded by Cancer Research UK, recruited over 4,000 women with HER2-positive early-stage breast cancer. It compared a six month course of Herceptin with the current standard of twelve months to see whether the shorter treatment was similar to or no worse than the longer treatment. This is the largest trial of its kind examining the impact of shortening the duration of Herceptin
The trial, led by a team from the University of Cambridge and the Clinical Trials Unit at the University of Warwick, involved 4088 women and is larger than any previous study in this field. It found that 89.4% of patients taking six months treatment were free of breast cancer after four years compared with 89.8% of patients taking treatment for twelve months. These results show that women who took Herceptin for six months fared no worse than patients who had standard 12 months treatment in terms of breast cancer returning. In addition, only 3% of women in the six month arm had to stop taking the drug because of heart problems compared with 8% in the 12 month arm. This trial mapped onto standard practice in the NHS where both chemotherapy and Herceptin are given before or after surgery.
Herceptin has been a major breakthrough, prolonging and saving the lives of women with breast cancers that carry the HER2 receptor on the surface of their cancer cells. Around 15 out of every 100 women with early breast cancers have HER2 positive disease. Herceptin is a targeted therapy that works by attaching to the HER2 receptors preventing the cancer cells from growing and dividing. Based on clinical trials a twelve month treatment course was adopted as standard with a requirement for 18 three-weekly injections. However, a further clinical study, the FinHer trial hinted that a shorter duration might be as effective, significantly reducing side effects and cost both to patients and to healthcare systems. The NIHR therefore funded this study to compare the standard 12 months of therapy with a shorter 6 month course.

Prof. Helena Earl
Lead study author Professor Helena Earl, Professor of Clinical Cancer Medicine, University of Cambridge and Cancer Research UK Cambridge Centre said “We would like to thank the 4088 patients who took part in our study and the tireless efforts and dedication of the trial teams at both Warwick and Cambridge. The trial would not have been possible without support from the NHS Clinical Research Network for the 152 teams at centres throughout the UK who recruited patients. In addition, the PERSEPHONE trials team has benefited enormously from an invaluable partnership with patient advocates throughout the study.
“The trial included patients who were receiving or going to receive Herceptin as standard in the NHS for HER2 positive breast cancer. The result indicates there are a large number of patients where a reduced Herceptin treatment duration of six months provides a similar benefit to 12 months without the risk of additional side effects. This data can now be added to all existing data on adjuvant Herceptin to be scrutinized by the wider breast cancer community for consideration of changes to practice. The study does however also suggest that there may be groups where the longer duration is needed to achieve maximum benefit. Women currently taking the medication should not change their treatment without seeking advice from their doctor. There is research to be done to define more precisely women who can reduce their treatment duration. We are poised to do important translational research analysing blood and tissue samples collected within the trial to look for biomarkers to identify subgroups where shorter or longer durations might be tailored.”
The trial has also collected qualitative research data on patient reported experiences on Herceptin. Common side effects reported in up to a third of women are aches/pains and fatigue with significant effects on daily functioning and quality of life. Cost savings for 6 months Herceptin compared with 12 months were presented at the ESMO conference in October 2018, and have been estimated at £9,699 per patient. Detailed cost effectiveness analysis including a life-time model, subgroup analyses and a societal cost analysis are underway. Given that HER2+ breast cancer represents a significant global burden of the disease, 6 months adjuvant treatment would translate into potential global savings of hundreds of millions of dollars annually. Worldwide this will have huge impact in middle and low income countries since it will facilitate Herceptin treatment (a World Health Organisation designated essential medicine) for many more women with HER2 positive early breast cancer.
Professor Charles Swanton, Cancer Research UK’s chief clinician, said: “Cancer Research UK’s work paved the way for the development of Herceptin, which has saved the lives of many thousands of women with breast cancer. But despite years of research, we haven’t been able to establish the optimal duration of Herceptin treatment, either to delay cancer coming back or to cure patients with early HER2 positive breast cancer following surgery.
“These eagerly anticipated results give the breast cancer research community an opportunity to reassess how long to give this targeted therapy to patients to see them living longer and with a better quality of life.
“The important next steps are to work out which patients can stop Herceptin at 6 months and which need extended therapy.”
Maggie Wilcox, President of Independent Cancer patients Voice (ICPV) who is the patient lead for the PERSEPHONE trial, said: “I am delighted to have been part of this landmark trial which is an important step to reduce the length of treatment whilst not changing effectiveness. Most trials add novel treatments to standard practice whilst this has set out to reduce duration of Herceptin. The collection of the patient reported experiences throughout the trial will greatly inform future practice and benefit patients. ICPV is working with the Persephone team to help disseminate these exciting results”.
Professor Janet Dunn who led the trial at Warwick Clinical Trials Unit said “The NIHR is a great funder for these types of trials as they ultimately refine treatment for patients with the maximum patient benefit ensured. Data collected on the patient reported experiences confirm the tough time patients have going through treatment and the impact any treatment has on their longer term quality of life”.
The results of the trial, PERSEPHONE, published in the Lancet today were presented at the June, 2018 ASCO Annual Meeting in Chicago, The full report, which will include analysis to determine the impact of treatment length on quality of life with patient reported experiences and a detailed cost effectiveness analysis, will be published in the NIHR journals library. Visit the project page for more information.
Written by CRUK Cambridge Institute
Virtual reality can spot navigation problems in early Alzheimer’s disease
Virtual reality (VR) can identify early Alzheimer’s disease more accurately than ‘gold standard’ cognitive tests currently in use, suggests new research.
The study highlights the potential of new technologies to help diagnose and monitor conditions such as Alzheimer’s disease, which affects more than 525,000 people in the UK.
In 2014, Professor John O’Keefe of UCL was jointly awarded the Nobel Prize in Physiology or Medicine for ‘discoveries of cells that constitute a positioning system in the brain’. Essentially, this means that the brain contains a mental ‘satnav’ of where we are, where we have been, and how to find our way around.
A key component of this internal satnav is a region of the brain known as the entorhinal cortex. This is one of the first regions to be damaged in Alzheimer’s disease, which may explain why ‘getting lost’ is one of the first symptoms of the disease. However, the pen-and-paper cognitive tests used in clinic to diagnose the condition are unable to test for navigation difficulties.
In collaboration with Professor Neil Burgess at UCL, a team of scientists at the Department of Clinical Neurosciences at the University of Cambridge led by Dr Dennis Chan, previously Professor O’Keefe’s PhD student, developed and trialled a VR navigation test in patients at risk of developing dementia. The results of their study are published today in the journal Brain.
In the test, a patient dons a VR headset and undertakes a test of navigation while walking within a simulated environment. Successful completion of the task requires intact functioning of the entorhinal cortex, so Dr Chan’s team hypothesised that patients with early Alzheimer’s disease would be disproportionately affected on the test.
The team recruited 45 patients with mild cognitive impairment (MCI) from the Cambridge University Hospitals NHS Trust Mild Cognitive Impairment and Memory Clinics, supported by the Windsor Research Unit at Cambridgeshire and Peterborough NHS Foundation Trust. Patients with MCI typically exhibit memory impairment, but while MCI can indicate early Alzheimer’s, it can also be caused by other conditions such as anxiety and even normal aging. As such, establishing the cause of MCI is crucial for determining whether affected individuals are at risk of developing dementia in the future.
The researchers took samples of cerebrospinal fluid (CSF) to look for biomarkers of underlying Alzheimer’s disease in their MCI patients, with 12 testing positive. The researchers also recruited 41 age-matched healthy controls for comparison.
All of the patients with MCI performed worse on the navigation task than the healthy controls. However, the study yielded two crucial additional observations. First, MCI patients with positive CSF markers – indicating the presence of Alzheimer’s disease, thus placing them at risk of developing dementia – performed worse than those with negative CSF markers at low risk of future dementia.
Secondly, the VR navigation task was better at differentiating between these low and high risk MCI patients than a battery of currently-used tests considered to be gold standard for the diagnosis of early Alzheimer’s.
“These results suggest a VR test of navigation may be better at identifying early Alzheimer’s disease than tests we use at present in clinic and in research studies,” says Dr Chan.
VR could also help clinical trials of future drugs aimed at slowing down, or even halting, progression of Alzheimer’s disease. Currently, the first stage of drug trials involves testing in animals, typically mouse models of the disease. To determine whether treatments are effective, scientists study their effect on navigation using tests such as a water maze, where mice have to learn the location of hidden platforms beneath the surface of opaque pools of water. If new drugs are found to improve memory on this task, they proceed to trials in human subjects, but using word and picture memory tests. This lack of comparability of memory tests between animal models and human participants represents a major problem for current clinical trials.
“The brain cells underpinning navigation are similar in rodents and humans, so testing navigation may allow us to overcome this roadblock in Alzheimer’s drug trials and help translate basic science discoveries into clinical use,” says Dr Chan. “We’ve wanted to do this for years, but it’s only now that VR technology has evolved to the point that we can readily undertake this research in patients.”
In fact, Dr Chan believes technology could play a crucial role in diagnosing and monitoring Alzheimer’s disease. He is working with Professor Cecilia Mascolo at Cambridge’s Centre for Mobile, Wearable Systems and Augmented Intelligence to develop apps for detecting the disease and monitoring its progression. These apps would run on smartphones and smartwatches. As well as looking for changes in how we navigate, the apps will track changes in other everyday activities such as sleep and communication.
“We know that Alzheimer’s affects the brain long before symptoms become apparent,” says Dr Chan. “We’re getting to the point where everyday tech can be used to spot the warning signs of the disease well before we become aware of them.
“We live in a world where mobile devices are almost ubiquitous, and so app-based approaches have the potential to diagnose Alzheimer’s disease at minimal extra cost and at a scale way beyond that of brain scanning and other current diagnostic approaches.”
The VR research was funded by the Medical Research Council and the NIHR Cambridge Biomedical Research Centre. The app-based research is funded by the Wellcome, the European Research Council and the Alan Turing Institute.
Written by the University of Cambridge
New diet and activity research collaboration tackles obesity and chronic disease challenge
The nation’s world-leading researchers in nutrition and physical activity have pledged to work together to tackle challenges associated with obesity and sedentary behaviours.
The UK is recognised internationally for the strength of its research in diet, nutrition, and physical activity. For the first time these experts from across the country have formally united to launch the NIHR Diet and Activity Research Translation (DART) Collaboration.
Professor Melanie Davies, Chair of the DART Collaboration and Professor of Diabetes Medicine at the University of Leicester, said: “Diet, nutrition, and physical inactivity underpin all the major chronic long-term conditions challenging the NHS. The relationship between food, nutrition exercise and health is complex, and is affected by biological as well as environmental, socioeconomic, cultural and behavioural factors. By pooling our collective expertise and through stronger cross-sector and cross-disciplinary partnerships we can tackle the major nutrition and activity research challenges facing our society.”
The DART Collaboration includes researchers from NIHR Cambridge Biomedical Research Centre, will focus on issues such as treating and preventing obesity in children and adults; nutritional phenotyping, including dietary assessment and stratification or personalisation of dietary advice; nutrition and aging; and the links between cardio-metabolic disease and obesity.
By developing a shared strategy, the DART Collaboration will maximise the impact of funding for experimental research in diet, nutrition, physical activity and sedentary behaviours. The Collaboration will agree priority areas, focusing on where there is the greatest unmet need or challenges and will collectively address these.
Dr Louise Wood, Director of Science, Research and Evidence at the Department of Health and Social Care, said: “Rising levels of obesity and diet-related conditions such as type 2 diabetes, cancer and cardiovascular disease, are putting a huge strain on patients, the NHS and the wider economy. The DART Collaboration is bringing together some of the best researchers in our hospitals and universities to work with charities and industry to drive innovation in approaches to diet, nutrition and physical activity in order to tackle this and benefit human health in the UK and globally.”
For more information about the NIHR Diet and Activity Research Translation Collaboration, email nocri@nihr.ac.uk.
About the NIHR Diet and Activity Research Translation Collaboration
The NIHR DART Collaboration includes researchers from across the NIHR’s Biomedical Research Centres:
● NIHR Bristol Biomedical Research Centre
● NIHR Cambridge Biomedical Research Centre
● NIHR Leicester Biomedical Research Centre
● NIHR Oxford Biomedical Research Centre
● NIHR Southampton Biomedical Research Centre
● NIHR Guy’s and St Thomas’ Biomedical Research Centre
● NIHR Imperial Biomedical Research Centre
● NIHR Newcastle Biomedical Research Centre
Clinical trial launch for new prostate biopsy device
A device developed by a Cambridge researcher to make prostate biopsies safer has begun clinical trials.
University of Cambridge and Cambridge University Hospitals (CUH) researcher and urologist, Mr Vincent Gnanapragasam (right), first developed the CamProbe as a prototype to take safer prostate biopsies in 2016. A major government grant enabled further development into a low-cost disposable device that can be used in any hospital outpatient clinic.
According to Cancer Research UK, prostate cancer is the most common cancer in men in the UK; in 2015, nearly 48,000 men were diagnosed with the disease.
The current method to retrieve samples from the prostate uses a transrectal ultrasound probe inserted into the back passage to allow the biopsy to be taken. Patients who undergo this procedure are at risk of urinary infections or sepsis as the needle has to pass through the bowel wall to reach the prostate. Around 30-40,000 prostate biopsies are done every year using this method in the UK alone.
The CamProbe has been designed so the biopsies can be taken more safely through the skin under the scrotum (transperineal) and avoiding the bowel. Mr Gnanapragasam, who also co-leads the Cancer Research UK Cambridge Centre Urological Malignancies Programme said: “The design of the CamProbe is a needle within a needle and allows us to collect tissue from the prostate through a more sterile part of the body. Most importantly it can be done under local anaesthetic in the outpatient department. Previously this kind of approach was only possible if a general anaesthetic was used with very significant additional costs”.
“The first CamProbe prototype, built in our engineering department, showed that we could reduce infections and sepsis to zero compared to rates of up to 12% with the current transrectal biopsy method. Now with this trial we will be testing the new and improved disposable version to ensure it performs just as well.”
Mr Gnanapragasam added: “Findings from our pilot study also showed most men preferred the CamProbe method over the current transrectal biopsy procedure.”
Clinical trials begin
The trial for the CamProbe is now underway using funding from the National Institute for Health Research (NIHR). It will run for a year at several hospitals around the UK including at the Cambridge Clinical Research Centre.
If the trial is successful, the CamProbe could be adopted into mainstream use within two years. Mr Gnanapragasam added: “We’re very excited clinical trials with our improved device are now underway. Our goal is to show that the CamProbe is a simple alternative for taking prostate biopsies which eliminates infection risks to patients and drastically reduces the need for antibiotics.
“The CampProbe’s simplicity also means it will be a low-cost device, and, in addition to reducing infections, the need for antibiotics and sepsis related admissions, could potentially save the NHS an estimated £7-11 million pounds every year. This will free up money for other healthcare needs.”
Recruitment target success in rheumatoid arthritis prevention trial
The research team behind a clinical trial to investigate a preventative therapy for rheumatoid arthritis are greatly encouraged after exceeding their patient recruitment target.
The study, supported by the NIHR Joint Translational Research Collaboration, had a target of recruiting 206 patients from hospitals across the UK and Netherlands, and this month they announced that they have successfully completed recruitment.
The randomised, double blind, placebo-controlled clinical trial aims to determine whether rheumatoid arthritis (RA) can be prevented if therapy is given to individuals at high risk of developing the disease. A patient is defined as high risk if there is a presence of autoantibodies in the blood, together with joint symptoms (pain but not joint swelling). Cambridge will be one of the BRC’s taking part in the study.
Professor Andrew Cope, Chief Investigator on the study and Professor of Rheumatology at King’s College and Guy’s and St Thomas’ NHS Foundation Trust, said: “This is a really tough study to recruit to because we don’t have access to existing cohorts of patients in the same way that we do for trials of established disease. These at risk subjects are referred by their GPs to early arthritis clinics cross the UK and the Netherlands. We then have to determine whether they fit the “at risk” phenotype. It’s tough on these at risk subjects too, because they are not only having to come to terms with being at risk of a chronic disabling disease, but then have to consider the risks associated with taking a preventative therapy – in this case weekly injections of a biological therapy for 12 months.”
The drug, called abatacept, is already licensed for use in patients with established rheumatoid arthritis. The study is investigating the feasibility, acceptability and effectiveness of a 12 month course of therapy with abatacept. The results from this trial will provide valuable insight into the “at risk” state and whether this intervention is effective. The study has benefited greatly from the support of many patients, patient focus groups and the National Rheumatoid Arthritis Society (NRAS), highlighting the enthusiasm and willingness of the Rheumatoid Arthritis community to explore new approaches to finding a cure. The researchers will also investigate immune and inflammatory responses before, during and after therapy with abatacept in order to better understand the immune system at the very earliest detectable stages of the disease.
Professor Cope said: “We chose abatacept because we know it has beneficial effects in patients with established rheumatoid arthritis, it has a good safety profile, and because of its beneficial effects on reducing harmful immune responses.”
Rheumatoid arthritis is a common chronic inflammatory disease that causes pain, stiffness, swelling and limited motion of joints. It can affect any joint (most commonly the small joints in the hands and feet) and can develop at any age. It is thought that around 400,000 people in the UK are living with the disease.
Diagnosis and treatment of rare diseases given boost through grant win
A project to help with the identification, diagnosis and treatment of the one in 17 people in the UK who have a rare disease in the UK has won a £400,000 grant.
Working with five NHS Trusts, the Rare Diseases Sprint Exemplar Innovation Project aims to develop a secure cloud research platform with the potential to transform the understanding of rare genetic disorders, drive improvements in diagnosis and provide proof of principle for use in other diseases.
The goal is not just to help patients but also help the NHS save money. The cost of an undiagnosed rare disease patient is significant – whilst undiagnosed, the cost per patient is more than twice that of other patients, an average difference of £7,000 more per patient per year.[1]
This project will be funded by UK Research and Innovation (UKRI) as part of the government’s Industrial Strategy and is a collaboration between Cambridge University Health Partners and Eastern Academic Health Science Network, Privitar, and AIMES, who will be working with NHS Trusts, and the National Disease Registries at Public Health England, Microsoft Research and the Wellcome Sanger Institute to build on the National Institute for Health Research’s (NIHR) investment in the NIHR BioResource for Translational Research in Common and Rare Diseases.
The aim is to build on the advances which clinical imaging, pathology and genomic technologies have made in understanding rare diseases by creating a secure, anonymised platform to draw together and integrate data from the NHS with research data.
The project will initially involve patients with rare diseases recruited to the NIHR BioResource – a national resource of volunteers who have already provided consent that information retrieved  from their health records can be used for medical research.
from their health records can be used for medical research.
Professor John R Bradley Director NIHR Cambridge Biomedical Research Centre and Co-Chair of NIHR BioResource said: “Rare diseases can be extremely difficult to diagnose because they often have an unidentified genetic cause. Recent advances in clinical imaging, pathology, and genomic technologies have led to remarkable progress in understanding disease – particularly rare diseases, but the power of these technologies cannot be fully realised until the immense volume of data generated can be integrated with NHS data, then analysed. This is what our project aims to achieve.
“We will only be using data from patients with rare diseases who have already consented to their information being shared for research. Using the expertise of the various partners involved, we will, in a secure environment that protects the privacy of individuals, link these patients’ NHS data with their genetic data and make this combined resource available for analysis as part of approved research studies. Creating this secure research environment has the potential to transform our understanding of rare diseases, help doctors to diagnose patients much earlier, and potentially unlock our understanding of how more common diseases work. This is clearly an exciting time for everyone involved.”
Debbie, a patient from London said: “As a patient with a rare disease, I think that this is a really exciting project that will make it much easier for researchers to work on rare diseases. To really understand a rare disease – what causes it and how to diagnose, treat or prevent it – researchers need access to as much relevant information as possible on as many patients as possible. I am reassured that there is a really strong emphasis on keeping our information secure and on privacy, to prevent individuals from being identifiable by researchers, and to make sure researchers are only given the information that they need. “
The project is one of ten innovative data solutions to healthcare challenges to receive a share of £3 million Government funding following a UK-wide competition. The initiatives will see NHS, universities and companies combining expertise and using health data responsibly to drive innovation and improve health outcomes for people across the UK.
Each of the initiatives will build on best practice and will inform the future delivery of a UK-wide infrastructure for health data research and innovation. This is the first step in creating ‘Digital Innovation Hubs’ across the UK to securely and safely connect data from the NHS with genomic data and other molecular data for research. Led by Health Data Research UK – the national institute for health data science – this will unlock opportunities for scientific discovery and support the development of future treatments, increase our understanding of disease, enhance health services and ultimately improve the way we are able to prevent, detect and diagnose diseases such as cancer, heart disease and asthma.
Professor Andrew Morris, Director of Health Data Research UK said: “These ten projects from across the UK, all led by clinicians working with researchers and industry partners, will demonstrate how the trustworthy use of health data and technology can improve patient pathways, make ground-breaking discoveries quicker and put the patient in charge. We are very excited about bringing these digital projects together with public participation and support so that health data research is brought to life at scale, demonstrating public and patient benefit of digital innovation in healthcare.”
Health Minister Nicola Blackwood said: “The NHS has an unrivalled data pool – we need to work with researchers, experts and industry partners to take full advantage of this to unlock solutions to some of healthcare’s biggest challenges.
“These ten innovative projects are just the start of a technological revolution to create one of the most advanced health and care systems in the world to diagnose diseases earlier, save lives and empower patients to take greater control of their own healthcare.”
[1] https://imperialcollegehealthpartners.com/new-report-reveals-undiagnosed-rare-disease-patients-cost-nhs-excess-3-4-billion/
Written by Cambridge University Health Partners







