‘Gene misbehaviour’ widespread in healthy population
NIHR Cambridge BRC researchers have been involved in a major study published this week in the American Journal of Human Genetics that shows that gene misbehaviour – where genes are active when they should be switched off – is a common phenomenon in the healthy human population.
Data Science and Population Health Theme Lead Professor Michael Inouye, fellow researchers Adam Butterworth, Emanuele Di Angelantonio, John Danesh and former colleague Dirk Paul at AstraZeneca took part in the research led by the Wellcome Sanger Institute that studied the activity of inactive genes in a large, healthy population for the first time.
While gene misexpression has previously been linked to several rare diseases, it is not known how often or why this may happen in the general population – but this study showed that misexpression is widespread across samples and involved more than half of the genes that should be inactive.
The surprising finding sheds new light on how our genetic code operates – and the approach could be used in future research to investigate, diagnose and develop treatments for various complex diseases caused by misexpression.
Study author Dr Katie Burnham at the Wellcome Sanger Institute said:
“The work of this pioneering large-scale study is testament to the incredible ‘genomics ecosystem’ in Cambridge that brought together experts from the Sanger Institute, the University of Cambridge and AstraZeneca.
“The findings open avenues for research into gene misexpression across different tissues, to understand its role in various diseases and potential treatments.”
In this study, researchers analysed blood samples from 4,568 healthy individuals from the INTERVAL study 3. They used advanced RNA sequencing techniques to measure gene activity and whole genome sequencing to identify genetic changes behind irregular gene activity.
Dr. Anne Forde, Patient and Public Involvement and Engagement Manager at the Cardiovascular Epidemiology Unit, University of Cambridge, said: “This research was based on the 50,000 population cohort recruited for INTERVAL on blood donation frequency, and we are grateful to the NIHR and the Cambridge BRC for your ongoing support and collaboration.”
- ‘Misexpression of inactive genes in whole blood is associated with nearby rare structural variants’, T. Vanderstichele et al. (2024), American Journal of Human Genetics.
Taking part in research: a patient’s perspective
When Mike Willis retired four years ago, he was looking forward to learning new skills and exploring new opportunities.
But a diagnosis of Motor Neurone Disease (MND) in 2022 forced Mike to revisit those carefully laid-out retirement plans and change direction. Read his blog to find out more!

“Remain mentally active.” That was the one thing I promised myself when I received my diagnosis of MND.
That was a particular challenge – I’m not a fan of crossword puzzles or anything like that.
But for the past ten years or so, I’ve been involved in Public and Patient Involvement (PPI) in clinical trials – work that I’ve been able to continue post-MND-diagnosis.
And I think it’s definitely helped keep the old grey matter ticking over!
My first foray into the world of PPI started ten years ago, when my GP asked me if I wanted to participate in the RAPSID study, investigating the use of peer-to-peer groups for the improved control of type-2 diabetes.
Following my retirement I joined several PPI teams for studies investigating weight loss and management to achieve remission of type-2 diabetes, at both MRC Cambridge (GLoW and SWiM) and Leeds Beckett University (Re:Mission).
I was also involved in the NERVE study sponsored by University Hospitals Dorset, evaluating a low-cost method to detect peripheral neuropathy in diabetes patients.
As I write this, I’m a member of the Cambridge University Hospitals PPI panel that reviews study proposals and documents, and more recently I’ve started reviewing project grant applications for the National Institute of Health and Care Research (NIHR).
Helping researchers find the best treatments and care
So why do I think people like you and me need to take part in research? Well, if you’re suddenly taken ill and rushed to hospital, or your GP refers you to a hospital clinic for further investigation, or you’re put on drugs to control a new condition, you want to know that you are getting the best possible treatment.
In fact, you can volunteer to help make sure that happens!
No new drug, procedure, test or treatment can be rolled out across the NHS without being clinically tested and proven. And to do that requires help from patients and members of the public.
Ways you can take part
- Volunteer as a patient
The input from patients and the public can be in a range of ways. The most obvious and well-known is to take part in a trial as a patient. This may happen if your GP Practice or Clinic is helping with a study and you are asked if you wish to take part, which is what happened to me 10 years ago!
Or you can visit the NIHR’s Be Part of Research website to see what opportunities there are and sign up for notifications for future research.
Nothing wrong with you? Lucky you, but in fact you can volunteer as a healthy person too, for comparison purposes in trials!
Each study has its own Patient Information Leaflet, explaining about the study, how much of your time will be needed, and any other details you may need.
All trials are vetted and approved by an ethical team, so rest assured you will be treated well and monitored carefully.
My experience of being on a study was interesting. The participants were randomised to different groups and we didn’t know what the expected outcome was, until the results were published.
- Volunteer as a public or patient member of a research team
Another way to volunteer is to actually be part of the research team.
“How can I do that, I’m not a scientist?” you may ask.
These days, all health-related projects have to include as part of their team members of the public who may have in the past or currently are experiencing the condition under evaluation. This is the Patient & Public Involvement (PPI) team and they help ensure the scientific team and project management focus on the needs of the patients.
Remember that Patient Information Leaflet mentioned just now, that all study patients receive? That will have been reviewed and edited by the project PPI team to make sure it is readily understandable and addresses a patient’s concerns.
The PPI team will do much more than that however – helping to write the project proposal, monitoring the project while it’s running, helping to prepare and review the many documents and reports while the project is in progress, and helping to disseminate the findings to clinicians, patients and the general public.
It’s really interesting to find out how health research projects are carried out, and so satisfying when the results are published!
I’ve just been part of a team evaluating a major NHS England trial, and I helped co-author one of the academic papers and I’m acknowledged on many of the others too!
- Volunteer to decide on research funding
The third way is to actually work for the NIHR, which is the government’s major funder of clinical research in England, as a volunteer reviewer.
We are allocated research proposals to review a few times a year. These detail the objectives of the research, the team that will carry it out, how the study will recruit patients and what they will do, how the results will be compiled and analysed, how the PPI team will be involved and how much it will all cost.
As a lay reviewer I’m sanity-checking the proposal from a patient perspective. Is the recruitment rate of participants realistic? Will they volunteer for the study or be put off by what they are asked to do? Is the PPI team really involved in the study, or are they there just to tick a box? And as one of my special interests, I look for EAU – Excessive Acronym Usage!
The nitty-gritty technical content of the proposal is peer-reviewed by scientists, but the lay reviewers can still contribute greatly to the assessment of each project.
After our reviews are submitted, the proposal will move onto a funding committee that decides whether it goes ahead, rejected or sent back for changes.
We get feedback of all of the review comments, so it’s really interesting to see how my comments and views on a study compare with others.
So there you have it! You can volunteer at all ages, and young people are under-represented so always welcomed.
There’s normally some financial compensation offered for your time; you won’t get rich but it’s a welcome sign that your time is appreciated and your contribution valued.
And if you do find yourself in a hospital clinic one day, you’ll be proud that the treatment you’ll receive has been developed in conjunction with volunteers like you!
Mike Willis
- To find out more about taking part in research, search #BePartofResearch on X (formerly Twitter).
Launched today! Phone app supports parents and carers of pre-term and sick babies
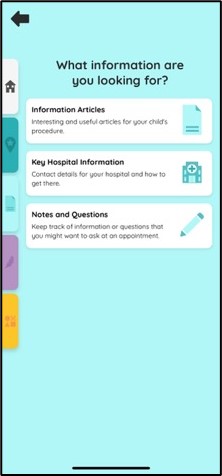
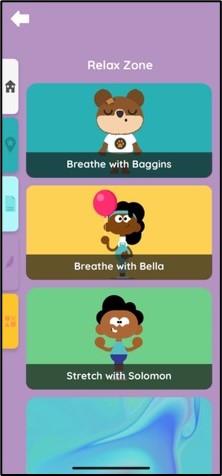
A mobile app launched today for parents-to-be and parents and carers with babies in the Rosie Hospital’s Neonatal Intensive Care Unit (NICU) could make visits less stressful – with a range of 360-degree visual tours, clinical information, relaxation techniques and even games for anxious guardians and siblings.
The Little Journey app was originally designed to help reassure children before they go to hospital for an operation, and has been specially modified to meet the needs of parents and carers with babies at NICU.

Consultant neonatologist and Director of the Evelyn Perinatal Imaging Centre, Professor Topun Austin (pictured) played a key role in the app’s modification: “Together with colleagues from East of England Neonatal Operational Delivery Network we received funding from the Health Foundation Q Community to look at ways digital technologies could help parents with babies on the NICU.
“We contacted NIHR Cambridge BRC to find out how best to get feedback and they facilitated a parent focus group, who loved the idea of an app designed to support parents and carers of babies in NICU.
“We then got in touch with Little Journey and they worked with families to develop the app tailored to the needs of parents and carers of babies on NICU.
“The result is amazing. We see this app as a digital ‘helping hand’, which can help reduce parents’ and carers’ anxiety, providing easily accessible information about where their baby is being looked after as well as clinical information relevant to their care.”
 |
 |
 |
 |

Award-winning innovation
The Little Journey app is one of several innovations selected by the NHS Innovation Accelerator initiative for its potential to enable patient and NHS staff benefit.
Prof Austin said: “Visiting hospital is anxiety-provoking for families, and the Little Journey app helps prepare them for their visits through fun, interactive and tailored-information that is always at their fingertips.
“This is a unique resource, with support and information for every stage of their child’s journey through NICU, from birth through to discharge and beyond.”
The information includes sections on neonatal care, common medical conditions and treatments, transfers to another NICU or local unit, going home, and palliative care and bereavement.
There are also virtual ‘360 degree’ tours of different parts of NICU and the PaNDR transport ambulance.
Prof Austin continued: “The app supports the whole family through what can be a stressful time with engaging content which informs, reassures and ultimately empowers them.”
Funding
Modification of the app was funded by the Health Foundation Q Community, the NIHR Brain Injury Med Tech Cooperative (now the HealthTech Research Centre for Brain Injury) and NIHR Cambridge BRC.
Roll-out
Currently the app is being rolled out across five hospitals in the East of England but the aim is for a regional and then national roll-out.
There is also scope within the app for it to be broadened to include clinical research projects. Prof Austin said: “We’ve seen how Little Journey can promote trial recruitment and cut early withdrawal, and this is a feature we would like to enable over time.”
Download Little Journey for the NICU

- Use your phone to scan the QR code to download the Little Journey app (for either iPhone or Android) and follow the on-screen instructions.
- Or visit: https://www.littlejourney.health/qr-scan
Prestigious lectureship award for NIHR Cambridge BRC stroke researcher
Congratulations to Dr Nicholas Evans, who has been awarded the prestigious Royal College of Physicians Linacre Lectureship.
The lectureship, which is awarded annually, is presented to a clinical academic under the age of 40 for their translational research in medicine.
Stroke Association Senior Clinical Lecturer and Honorary Consultant Dr Evans will deliver his lecture “Advances in stroke medicine: blood vessels, brains and beyond” at the Royal College of Physicians on Monday 25th March.
Dr Evans said: “The lecture will focus on advances in stroke medicine over the last 20 years and the translational work being done in Cambridge and the region.
“Those advances have been really quite amazing.
“My grandmother had a stroke in the 1990s and she didn’t recover. Now we’re seeing people who have had a thrombectomy following stroke walking out of hospital within days, and they are making excellent physical recoveries.
“My research looks at what happens when carotid arteries (the blood vessels to the brain) fur up – this is atherosclerosis and it’s a major cause of stroke.
“But we need to look more closely at the effects downstream on the brain from this inflammation, this furring up of arteries.
“Understanding this interaction between what’s happening in the blood vessels and brain health may help provide new therapeutic approaches for reducing the effects of stroke.
“Finally I’d like to thank NIHR Cambridge BRC for their huge support to my work and the research of my colleagues in stroke, imaging and cardiovascular medicine.
“They have certainly made a difference.”
- Every year around 100,000 people in the UK will have a stroke. Stroke is a leading cause of adult disability, and cerebrovascular disease more broadly is a major cause of dementia.
- “Advances in stroke medicine: blood vessels, brains and beyond” Linacre lecture 2024 is being live-streamed from the Royal College of Physicians and is open to all members (registration free). For more information visit this link.
Infants with brain injury may face social/emotional and behavioural complications, NIHR Cambridge BRC-supported study suggests
Babies who suffer brain injury before, during or shortly after birth because of lack of oxygen to the brain may face longer-term socioemotional and psychological complications, research supported by the NIHR Cambridge BRC has shown.
The research, “Socioemotional and Psychological Outcomes of Hypoxic-Ischemic Encephalopathy: A Systematic Review”, is a systematic review of the literature led by PhD student Grace Kromm and is published in the prestigious journal Pediatrics.
Grace’s supervisor and Consultant Neonatologist at Cambridge University Hospitals Professor Topun Austin said: “Hypoxic ischemic encephalopathy, or HIE, is an umbrella term for a brain injury that occurs when oxygen or blood flow to the brain is reduced or stopped.
“It can be serious, leading to life-long disabilities and even death – although both these have been reduced with the use of therapeutic hypothermia on babies with moderate to severe HIE.
“Clearly with HIE the immediate short-term goal is the infant’s survival, and so far, research on disability after HIE has focused on early motor or cognitive deficits. But this runs the risk of neglecting longer-term social/emotional and psychological/psychiatric outcomes for these infants.
“This systematic review shows there is a growing body of literature reporting statistically significant associations between these outcomes and HIE, not just in the first few years of an infant’s life but into their adolescence and even adulthood.”
Literature review
The research team undertook a comprehensive systematic review of the literature looking at HIE-related studies in major health-sciences databases, including the Cochrane Library. Over 3000 papers were screened and eventually the research focused on 43 studies representing 3244 HIE participants and 2132 comparison participants, mostly in Europe and the US.

The review showed a clear social, emotional, and psychological burden after perinatal HIE, which persisted in many cases into adolescence and adulthood, even when excluding children with other disabilities such as cerebral palsy.
These statistically significant differences included worse social/emotional and psychological outcomes after HIE, with even mild HIE being associated with lower health-related quality of life and behavioural problems.
Moderate-severe HIE was associated with disrupted personal-social skills and development, behaviour problems and a higher incidence of psychiatric diagnosis, including diagnoses of autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) as well as sleep problems like sleep anxiety.
The review also showed that some socioemotional and psychological complications increased as children aged, suggesting that children who experienced perinatal HIE would benefit from long-term follow-up.
Longer-term support for children with HIE
Grace Kromm said: “As the sister of a young adult who experienced perinatal HIE, I have witnessed firsthand how difficult behavioural challenges such as these can be for the individual and for caregivers.
“This review emphasizes the importance of early and longitudinal intervention across socioemotional and behavioural domains, even for children with mild HIE and generally positive prognosis otherwise.
“Additional research should focus on possible mediators of outcome as well as early screening tools to identify children at highest risk as early as possible.”
Professor Topun Austin said: “Well done to Grace, her research students at Robinson College, Hilary Patankar and Shubang Nagalotimath, and research advisor Dr Hilary Wong for this important work!”
How genetic testing answered questions for local family living with a rare disease
Baby Louisa was about six weeks old when her mum Michaela started to be concerned about her.
At the time, the family were living in Canada and Louisa – who was born with an ectopic kidney – wasn’t feeding and wasn’t putting on weight.
Michaela said: “She also looked a little different. But nothing was screaming out at me. Just a lot of little things which were worrying.”
When Michaela took Louisa to the emergency department with breathing problems – by now her breathing was very laboured and noisy – she was told by staff that Louisa’s oxygen levels were fine and that this was just something her daughter would outgrow.
Michaela continued: “So I thought, okay, she’s with an ectopic kidney but she can live without a kidney, she has this airway disorder but she’ll grow out of it. But she reached about three months old and she still wasn’t gaining weight, at four months she still looked like a newborn, by eight months she wasn’t sitting.”
“It’s been transformational”
On a prolonged stay in the UK, Michaela took Louisa to Great Ormond Street Hospital, where she was referred to a neurologist. Michaela said: “He said to me, please don’t listen mum, because I’m going to report the things that I see.
“He then highlighted all the things he noticed about how Louisa looks, saying her ears are too low, her lips are too thin, her nose is too flat. He listed all the visual parts of her that suggested to him that she has a genetic condition.
“That hadn’t even been on my radar. But I remember being really upset, because this was my beautiful baby he was talking about. Everything that we think is beautiful about her, is actually something that could be considered a genetic feature. So we think her eyes are extraordinary, the most beautiful part of her, but they’re also an indication of something else.”
On the family’s return to Canada, doctors recommended genetic testing for mum, dad and Louisa.
The results showed that Louisa had genetic variants including one de novo genetic mutation in ZNF865, but no one knew what it meant. At the time, doctors knew of just one other child from Denmark who had the same gene mutation. (There are now 13 children worldwide recognised with the same diagnosis.)
Michaela said: “I remember being really disappointed when the results came through, because I had expected answers, what did Louisa have? How long would she live for? How can I best help her?
“But having those few letters and numbers was transformational, we just didn’t realise it at the time.”
Community
In 2019 the family moved back to the UK, Louisa was placed under the care of Dr Marlow, consultant paediatrician at Colchester Hospital, and the results of her genetic tests in Canada were shared with the NHS.
Former primary-school teacher Michaela is now full-time carer for Louisa, who along with other children with the same mutation, has global developmental delay and a variety of health problems caused by the change in the ZNF865 gene. She has a severe sleep disorder and is non-verbal.
Michaela believes the genetic tests for her daughter have given them above all a community: “If you have a disabled or severely disabled child without a diagnosis, you can find yourself in a no man’s land and that’s really scary and lonely.
“I don’t feel I’m there anymore because thanks to genetic testing I have part of an answer, a combination of letters and numbers, and all of a sudden our world opened up to more people. That’s been emotionally invaluable.”
Geneticists have received funding to research the condition and the next step is to get it officially recognised as a rare condition.
Michaela said: “We don’t know what her future holds, but we do know that every day she is a blessing to us. She shows us the way.”
How a collaboration is helping more families in rural and deprived areas to access WGS tests
Michaela and her family are very grateful to have had whole genome sequencing for her daughter – and now more children with neurodevelopmental disorders (NDDs) can have access to whole genome sequencing tests, thanks to a collaboration between the Synapse Centre for Neurodevelopment in North Essex and NIHR Cambridge Biomedical Research Centre.
In fact, in just six months the collaboration has shown dramatic results.
Dr Marlow (photo, below), Director of the Synapse Centre said: “One in five of the children I see has a moderate-to-severe learning disability, or NDD, and genetic testing is essential to help us understand them biologically and genetically.

“But until recently we simply didn’t have the capacity to recruit and consent patients and families for genetic testing and research.”
This changed in May last year when the Synapse Centre joined forces with NIHR Cambridge BRC’s Antenatal, Maternal and Child Health theme; one of the resources it is funding is dedicated research time to consent families for whole genome testing.
Dr Marlow said: “It takes up to an hour to consent each family, and in a service which is already pressed clinically it’s really important to protect this time, which BRC funding has enabled us to do.”
The results speak for themselves. From May to November last year, the Synapse Centre recruited 27 families for WGS testing (compared with just two families in the previous 12 months) and almost 60 children to take part in research studies related to their conditions.
Dr Marlow said: “WGS tests are a vital part of our work at the Synapse Centre, which researches conditions such as autism, ADHD, Cerebral Palsy and genetic syndromes.
“The tests not only provide more accurate diagnoses, they could also open up more personalised interventions and treatments to help meet the needs of children with these conditions, both now and in the future.”
Antenatal, Maternal and Child Health Theme lead Professor David Rowitch said: “For patients with rare genetic conditions, we can offer tools for rapid diagnosis using whole genome sequencing, and we’re committed to providing these services across our region including under-served areas such as Colchester.
“Our studies, inclusive of diverse populations, will better demonstrate the value of advanced diagnostic services across NHS England.”
Recruiting to studies
Through the collaboration the Synapse Centre has recruited nearly 60 children to clinical trials, including the Cambridge-led NeuralNET Cerebral Palsy pilot study which is looking at the feasibility of WGS tests for children with CP whose clinical care might be changed by the result.
Dr Marlow said: “Studies have shown up to a third of children with cerebral palsy potentially have a genetic reason for their condition. This opens the door to potential treatment options that were not available before and we can address symptoms earlier.
“And if we can address them early on, we can prevent them from progressing which can lead to further complications.”
Overcoming barriers
Funding from the collaboration with NIHR Cambridge BRC is also paying for a paediatrics genetics counsellor for Colchester Hospital – which currently has none – who will start in early 2024.
Dr Marlow said: “This is badly needed and will go some way to addressing inequality of access to genetics services in the region.
“Part of this role will include patient recruitment to genetic research studies and to the NIHR BioResource.
“Having a paediatric genetics counsellor in situ will also help in promoting the benefits of WGS in the region, where under-served communities in particular face a combination of economic, social, environmental and cultural barriers.
“For example families may live in very deprived communities, or their parents have similar learning challenges, or they don’t access healthcare, or their sociocultural and physical environment may determine what’s acceptable to them.”
Framework for district generals
The collaboration with NIHR Cambridge BRC could serve as a framework for other district general hospitals across the East of England.
Dr Marlow added: “Our collaboration with Cambridge has accelerated our whole genome testing across paediatrics, but particularly in NDD.
“It’s also helped connect local families with the genetic service that is trying to define their needs better, and it’s raised the need to prioritise early intervention through better understanding of biological difference to the local ICB (Integrated Care Board) that commissions healthcare services for our population.
“And it’s shown how district general hospitals – which tend to offer fewer WGS services – can work with major university centres to promote WGS tests that aren’t universally commissioned but which are fundamental to helping children with additional needs.”
Professor Miles Parkes, Director of the NIHR Cambridge BRC, added: “It is great to see this cutting-edge research being taken out across the region and to see the enthusiasm with which it is being embraced in Colchester.
“It is critically important that our BRC-funded research infrastructure reaches into all corners of the region so that its benefits can be distributed beyond the traditional big university centres.
“We are very grateful to the team in Colchester for supporting the work and we very much hope that this will be a springboard to future collaboration.”
Personal motivation
Dr Marlow also has a personal motivation driving his research. His nine-year-old son Freddie is non-verbal, autistic and has many health issues.
Dr Marlow said: “In 20, 30 years’ time I firmly believe we’ll have a genetic and biological reason for his brain development, and that moreover every child in the UK will have their genome sequenced at birth, as the start of individualised care that will last their lifetime. “We’re not there yet, but we have to start somewhere and widening access to WGS testing is an important step to better understand and help children with NDDs, for children like Louisa and Freddie.”
- 29 February is Rare Disease Day. Visit their website to find out more about this incredible grass-roots globally movement on rare diseases.
- It’s not too late to join our Rare Disease webinar on patient-centred research! Book your ticket on EventBrite before 12pm today.
Inspire Inclusion: research nurses discuss their careers for International Women’s Day
International Women’s Day (IWD) is celebrated worldwide on 8 March each year – and the theme for this year is ‘Inspire Inclusion.’
We asked our NIHR Cambridge Clinical Research Facility research nurses in the Cambridge Clinical Research Centre (CCRC) what inspired them to choose research as a career path – and why inspiring women to work in research leads to more empowerment for them, better research, and a better world.
Each podcast is less than 20 minutes – and a powerful reminder that IWD belongs to everyone, everywhere! The host for both podcasts is our own Patient and Public Involvement Coordinator, Georgina Norris.
- PODCAST: “Desire to be part of something bigger”
- Diana is Senior Research Nurse at the NIHR Cambridge Clinical Research Facility, and Rosemary and Cyra are Research Nurses. In this episode Georgina asks them how they first got involved in research, from a curiosity to improve healthcare, to a chance conversation with a colleague, to a research module as a student. Georgina then asks Diana, Cyra and Rosemary what they love about working in research – and what can be done to inspire more women to work in the field.
- Download the transcript.
- PODCAST: “Research gives a lot of people hope”
- Joanne is Workforce Skills lead for the NIHR Cambridge Clinical Research Facility, and Karolina is a Research Nurse. In this episode Georgina asks them how they first got involved in research, from a curiosity to improve healthcare, to deciding to just ‘give it a try’. Georgina then asks Joanne and Karolina what they love about working in research.
- Download the transcript.
Inspired to find out how you can get involved in research?
If you’ve been inspired to find out more about how you can get involved in clinical research, we have a lot of information and signposting on our website.
- If you’re a hospital patient or member of the public, visit our PPIE web pages for information on how to take part in research and how to join the CUH PPI Panel.
- If you’re a healthcare professional working on the Cambridge Biomedical Campus, visit our Training & Professional Development pages for signposting to research training and opportunities.
No simple choices: tickets now open for our Cambridge Festival panel discussion on privacy and consent in health data research
How is health data used for research? What choices do researchers make when using patient data for their research?
And what impact do those choices have not only on the quality and reach of their research, but also on patient privacy and public trust?
At this free virtual event, researchers Professor Angela Wood, Dr Raj Jena and Dr Ari Ecole will join patient advocate Rosanna Fennessy as they look at four real-life examples of local research where health data was used.
The panel will then explore the challenges and concerns that emerge from different types of data use – followed by a Q&A from the audience logging in online.
Book your tickets on EventBrite
Join us online on Thursday 14 March 2024 from 5.30pm to 7.00pm. Make sure you don’t miss out: visit our Eventbrite page to reserve your spot.
This event is part of this year’s Cambridge Festival. We will also be at the Cambridge Academy of Science and Technology for the Festival’s family weekend on 16 March – put it in your diary now!

Other events from NIHR organisations
Find out what our partner NIHR organisations are also doing at the Cambridge Festival
- What INTERVAL is right? Racing like a blood donor
Saturday 16 March 9.30-16.30 at CAST, Cambridge Biomedical campus
An interactive stall to engage young people and families in learning about blood donation research.
- Inheritance, DNA, and Heart Disease
Schools day Tuesday 19 March 10.00-14.00, West Cambridge Hub.
A craft workshop which will use beads and elastic to make bracelet models of DNA. The children will then use those models to learn how to detect bad characteristics and what scientists are doing at the University of Cambridge to understand and prevent diseases such as heart disease. - Artificial Intelligence, with great power comes great responsibility
Tuesday 19 March – 5:00pm-6:00pm or 6:30pm-7:30pm at Babbage Lecture Theatre, New Museums Site Downing Street, CB2 3RS
An interesting talk exploring what is Artificial Intelligence and how it works.
- Who has the healthiest heart?
Sunday 24 March 09.30-16.30, University of Cambridge Student Services, New Museums site
A family-friendly fun card game to learn all about cardiovascular disease risk prediction and how it can identify adults at risk of heart disease. Make sure you book your place to attend.
- 12:00pm-1:00pm on Tuesday 26 March
- 5:00pm-6:00pm on Tuesday 26 March
- 12:00pm-1:00pm on Wednesday 27 March
- 5:00pm-6:00pm on Wednesday 27 March
The NIHR BioResource’s DNA, Children + Young People’s Health Resource (D-CYPHR) programme are inviting children and their parents to learn about the power of spit to impact health research.
About the NIHR
The National Institute for Health and Care Research (NIHR) is the UK’s largest funder of health and care research. Its mission – to improve the health and the wealth of the nation through research – is shared across all its constituent programmes, units and centres.
If you’d like to find out more about how the NIHR works, visit www.nihr.ac.uk. To find out how YOU can be a part of it, go to bepartofresearch.uk
Candidates sought for NIHR senior leadership posts
Do you want to work for an outstanding organisation at the forefront of Health and Care research delivery?
The National Institute for Health and Care Research (NIHR) is seeking individuals with extensive experience of senior leadership within a health or care specialty or setting for 30 National Specialty Lead and four National Setting Lead part-time roles to act as high calibre ambassadors for the NIHR Research Delivery Network and to provide senior leadership and strategic direction for matters of research delivery across the relevant specialty and setting.
The roles are available in the following Specialties and Settings on a 0.1 Full-Time Equivalent (FTE) basis (more FTE may be available for several posts as detailed in the further particulars):
Specialties:
Ageing; Anaesthesia, Perioperative Medicine and Pain Management; Cancer; Cardiovascular; Children; Critical Care; Dementia & Neurodegeneration; Dermatology; Diabetes, Metabolic & endocrine; Ear, Nose & Throat; Gastroenterology & Hepatology; Genetics; General Practice; Haematology; Imaging; Infection; Mental Health; Musculoskeletal & Orthopaedics; Neurology; Ophthalmology; Oral & Dental; Palliative Care; Public Health; Renal; Reproductive Health & Childbirth; Respiratory; Social Care; Stroke; Surgery; Trauma & Emergency Care.
Settings:
Hospitals; Primary Care; Community-Based; Residential Care.
These high-profile national roles will report into the RDN Strategic Development Director and form part of the RDN National Specialty and Setting Leadership team, and will proactively develop effective relationships with decision-makers in the public sector, charity and life science organisations (non-commercial, pharma, medtech, diagnostics and biotech) and other related external stakeholders to further the aims of the NIHR RDN. The posts will commence from April 2024.
To apply please submit a concise CV and a supporting statement (maximum of three pages of A4, font size 12) setting out your vision and suitability for the role. The deadline for applications is midnight on Sunday 25 February and virtual interviews are anticipated to take place during the period 11-22 March.
For further information about these roles and to submit an application, please visit the University of Leeds website or for any queries please contact NSSLappts@nihr.ac.uk.
New trial brings screening for oesophageal cancer closer
A man from Cambridge is the first to join the surveillance part of a clinical trial part-funded by NIHR Cambridge BRC and supported by the NIHR Cambridge Clinical Research Facility, that could see routine screening for oesophageal cancer introduced into the NHS, potentially halving deaths from this cancer every year.
The capsule sponge, known as the pill-on-a-thread, is a quick and simple test for Barrett’s oesophagus, a condition that can be a precursor to cancer. Heartburn is a common symptom of Barrett’s oesophagus, a changing of cells in the food pipe.
The BEST4 trial launched at Addenbrooke’s today is the final step to see if the capsule sponge can prevent oesophageal cancer when used to screen or monitor those most at risk of the disease. If so, it could become a national screening programme across the NHS, in the same way mammograms are used to screen for breast cancer.
The first stage of the trial, BEST4 Surveillance, is for people already diagnosed with Barrett’s oesophagus. It will look at whether the capsule sponge test could replace endoscopies to monitor their condition. Participants will receive both examinations during the trial with results used to assess their risk of developing oesophageal cancer.
The second stage of the trial, BEST4 Screening, opens in the summer and will recruit 120,000 people aged over 55 on long-term treatment for heartburn.
The multi-million-pound trial is jointly funded by Cancer Research UK and the National Institute for Health and Care Research.

Tim Cowper, 49, a brewer from Cambridge (pictured), has had acid reflux, or heartburn, every night since he was 16. A routine health check while he was at university resulted in the shock diagnosis of Barrett’s oesophagus. After his diagnosis, he has been monitored ever since.
Tim said: “I was alarmed when I was told that having Barrett’s meant having pre-cancerous cells in my gullet. Cancer is never a nice word to hear, especially when you are so young, but luckily, I’ve had my condition monitored.
“Since my diagnosis, I’ve been going for an endoscopy at least once every three years to monitor my oesophagus. It is not pleasant at all. Each time I have a thick tube pushed down through my mouth and I can feel every single one of the biopsies taken by the camera. Swallowing a capsule sponge is a much better experience and I now get the test before my regular endoscopy appointment.”

The trial builds on decades of research led by Professor Rebecca Fitzgerald (pictured), a doctor and researcher at Addenbrooke’s and the University of Cambridge. She and a team of scientists, clinicians and nurses at the Early Cancer Institute, University of Cambridge and Cancer Research UK Cambridge Centre, invented and refined the capsule sponge test.
Prof Fitzgerald said: “The capsule sponge, a quick and simple test for Barrett’s oesophagus, could halve the number of deaths from oesophageal cancer every year. Cases of oesophageal cancer have increased six fold since the 1990s. On average only 12% of patients live more than five years after diagnosis. Most don’t realise there’s a problem until they have trouble swallowing. By then it is too late.
“The first phase of the trial looks at whether the capsule sponge can be used as a cancer early warning system for patients diagnosed with Barrett’s. Using the capsule sponge and a new set of lab tests, we will be monitoring patients to see if we can prevent more cases of cancer.”
Barrett’s oesophagus is currently identified via an endoscopy and a biopsy in hospital following a GP referral. It is time-consuming, unpleasant, and quite invasive for patients, as well as being expensive for the healthcare system.
The capsule sponge is a small, easy to swallow capsule on a thread, which contains a sponge. The patient swallows the capsule which dissolves in the stomach and the sponge expands to the size of a 50p coin.
The sponge is carefully pulled back up using the string, collecting cells for laboratory testing. The test takes just 10 minutes and can be done in a GP surgery.
Cancer Research UK and others have funded several successful clinical trials to demonstrate that the test is safe, accurate and can detect 10 times more cases of Barrett’s oesophagus than standard practice.
The test is faster and cheaper than endoscopy, which is currently used to diagnose and monitor Barrett’s oesophagus and oesophageal cancer. It has been piloted in health services in England, Scotland and Northern Ireland for patients who are currently on waiting lists for endoscopy because they have long-term heartburn or diagnosed with Barrett’s oesophagus.
Executive Director of Research and Innovation at Cancer Research UK, Dr Iain Foulkes, said: “Around 59% of all oesophageal cancer cases are preventable. Yet endoscopy, the gold standard for diagnosing and treating this cancer, is labour-intensive. We need better tools and tests to monitor people most at risk.
“Backed by funding from Cancer Research UK, the capsule sponge has become one of the most exciting early detection tools to emerge in recent years. It’s a remarkable invention by Professor Fitzgerald and her team, and previous trials have shown how powerful it can be in identifying cancer earlier.
“There are 9,200 people diagnosed with oesophageal cancer in the UK every year and the capsule sponge will mean they can benefit from kinder treatment options, if their cancer is caught at a much earlier stage.”
The future Cambridge Cancer Research Hospital will bring together clinical and research expertise, including Professor Fitzgerald’s work, under one roof. It will enable the development and discovery of more non-invasive devices like the capsule sponge, to detect cancer earlier, and save more lives.
Tim said: “I’m really lucky as my own condition hasn’t got worse and there are no signs of progression to full cancer. I take care with my diet to keep my acid reflux in check, but I can still have the odd curry and, most importantly, taste the beer I make!
“Taking part in this study means a lot to me. My condition was caught before it even became a fully-fledged cancer. Sadly, many others aren’t so lucky. The capsule sponge could help others whose acid reflux is causing something more sinister.”
The BEST4 Surveillance Trial is led from Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, with trial design, coordination and analysis of results by the Cancer Research UK Cancer Prevention Trials Unit at Queen Mary University of London. More information about the BEST4 trial can be found at www.best4trial.org
Paediatric cancers: a researcher’s quest to find answers
Clinician, researcher, teacher, mother: it’s exhausting just listing the many hats that paediatric consultant oncologist Dr Aditi Vedi wears.
But we interviewed Aditi not to find out how she ended up in Cambridge UK, 12,000 miles away from her home in Sydney, nor how she raised three young children while working on her PhD (although these are also fascinating!)
Instead we wanted to find out more about her two main research projects that NIHR Cambridge BRC is funding.
It started with a question that Aditi asked herself: What does chemotherapy do to stem cells?
Aditi explained: “We know that children who have chemotherapy are at increased risk of leukaemia, diabetes, metabolic disease and heart disease in the future.
“But just recently, in the space of a year, two children who had received chemotherapy for other reasons were back in hospital because they had developed leukaemia.
“I looked in the scientific literature and, although rare, there are reported cases of this happening and no one really understands why.
“That got me thinking, can I build on what I know about stem cell behaviour from my PhD and look at what chemotherapy does to those stem cells in children?”
In her PhD, Aditi looked at how genetic mutations affect blood stem cell development in adult leukaemia patients.
She found that the gene mutation DNM3TA blocks the production of healthy blood stem cells in adult patients, which then predisposes them to leukaemia.
Now working in paediatric oncology, Aditi decided to build on her knowledge of stem cell behaviour to see how they behave in children with all types of cancer who have received chemotherapy.
That led to the research project on therapy-related AML in children, , in which Aditi will study the effects of gene mutations in blood stem cells from children who developed leukaemia after getting chemotherapy for another cancer. This is in collaboration with Dr Alex Thompson from Nottingham University and is part-funded by the NIHR Cambridge BRC and the Little Princess Trust.
At the same time that Aditi was setting up this trial, she was working on another to investigate making whole genome sequencing (WGS) faster. If successful, this trial has the potential to change clinical practice in England.
Aditi said: “WGS has changed clinical practice for many paediatric cancers, and the NHS has now commissioned this test for all children and young adults up to 25 with cancer or relapsed cancer.
“But it can take up to three months to get the results back. And for aggressive cancers like leukaemia or metastatic disease that’s often too late – and doctors have to make clinical decisions before the data’s back.”
So Aditi is working with current NHS WGS supplier Illumina on a trial to develop a pipeline to deliver WGS data in 24-48 hours.
The Ultrafast WGS study has already recruited 11 patients, and preliminary results are so promising that Aditi has applied for more funding from a number of organisations including Addenbrooke’s Charitable Trust and the Rosetrees Trust to expand the study to 100 patients, whom she hopes to recruit throughout 2024.
Aditi explained: “This is achievable, especially if we include another site and several other hospitals have approached me about joining the study, including Great Ormond Street Hospital who are already very proactive about recruiting patients into WGS.
“If we can get to 100 patients then that would hopefully provide enough data to clearly demonstrate that not only is it feasible but superior to the current NHS pipeline.
“And it could replace not just the WGS pipeline but also other tests including DNA and RNA sequencing, that the NHS currently pays for separately as needed.
“It may work out cheaper to run the single new test rather than up to five smaller tests.”
While the sequencing is the most expensive part of the trial, the funding from NIHR Cambridge BRC covers staff time, including the appointment of a genomics nurse and dedicated research time for Aditi and the geneticists involved.
Next steps
Both projects are expected to take about three to four years. After that, Aditi plans to undertake more discovery and translational science: “My passion lies in acute myeloid leukaemia (AML), which is less common than lymphoid leukaemia in children, but much more aggressive, and which hasn’t really had any major new treatments over the last two decades.
“I’d like to work with other scientists to look at how AML develops in children, and how we can target its weaknesses with newer medications.
“Research is so rewarding, it gives me a purpose for what I do in the clinic, to look for the answers to the questions that directly relate to my patients.
“That’s amazing.”
- To celebrate the work and impact of the NHS research workforce, the NIHR ran its Shape The Future campaign throughout November – find out more here: https://www.nihr.ac.uk/explore-nihr/campaigns/nhs-75/
Research nurses “the key to study delivery”
The publication of a small research study completed during his Master’s in the early noughties was enough to give Dr Adrian Boyle the research bug.
The Consultant in Emergency Medicine at Addenbrooke’s said: “It was a small study and in fact not even very exciting, but it made me want to discover new ways of looking after patients.
“I saw the benefits of research on patient care, and wanted my department to take part in big multi-centre trials.”
His initial attempts met with failure: “In 2005, the international study CRASH-2 opened, looking at the effect of the generic drug tranexamic acid (TXA) on mortality in bleeding trauma patients.
“I wanted our department to join but the logistics were too difficult, the processes were too complicated, and this really important study was done without us.”
Launch of NIHR
Then in April 2006 the NIHR (which was then called the National Institute for Health Research) was created under the government’s health research strategy, Best Research for Best Health.
Adrian said: “The launch of the NIHR and its funding for dedicated research nurses was a game-changer.
“Yes, it took some time to embed our research nurses and initially they were employed on short-term contracts.
“But we got interesting results from the studies we took part in, which built up our reputation.
Changing standard of care
“Then when CRASH-3 started in 2012, we were in a position where we could take part, and we recruited more than 150 patients, one of the best recruiting sites worldwide.
“This trial showed that giving TXA to people with head injuries actually saved lives.
“As a result this is now a standard of care around the world, and in the UK TXA is given to everyone with a major head injury.”
Embedding a research team
The Emergency Department at Addenbrooke’s currently has three research nurses, with a combined working full-time equivalent of 2.2 nurses.
Kerry Meynell is one of the research nurses. She said: “I love the research we do, and what we achieve, and working in a team who are all trying to make a difference to healthcare.” The role’s flexibility is also a big plus for Kerry: “My husband’s in the army and we have three children, so I really need a role that not only gives me job satisfaction but also work/life balance so I can fit it around my family life.”
Another key member of the team is the administrator, Audrey.
Kerry said: “With research there’s a huge amount of paperwork, record-keeping and associated tasks to complete. The research nurses used to do them all but having Audrey on board means we can spend more time on educating staff and patients/families about the research and on recruitment.”
Adrian added: “When I look back over the last two decades, research delivery has improved beyond all recognition. When I started as a consultant at Addenbrooke’s, it was very much a case of here’s a study, here’s some money, now go and recruit a research nurse to do that study.
“And this was a really time-consuming, inefficient process.
“Now we have support from embedded research nurses, we also have regular access to our local NIHR Portfolio Support Manager, who’s been invaluable in terms of planning and supporting our studies.
Successful model
“We’ve shown the model works. You don’t have to shift the dial very much in terms of either the money or the support to create an embedded team in the clinical team.
“And that leads to more research capability. In the emergency department we’ve grown from having just one or two studies open to six to eight studies open at the same time, including a head injury study, chest studies around pneumothoraces and an infection study screening for severe infections.
Preventative research
The research that takes place in the Emergency Department is not all to do with major health events such as trauma, heart attacks and strokes; it can also be preventative and the embedded research nurses are ideally placed for this kind of research. Kerry explained: “One of the first trials I worked on was the CoSTED trial. While patients were in the waiting room, I approached them to discuss their smoking status.
“It was a good opportunity to prompt positive behaviour change. The patient’s in Emergency Department, they’re thinking about their health and the consequences of their lifestyle, so it can be an ideal time to talk about stopping smoking and the help which is available.”
Transforming research delivery
Adrian said: “The step change in research delivery has been a career highlight for me.
“We’re now at that level of organisation and support where we can run big studies very quickly and efficiently, as was seen in the pandemic, when we ran diagnostic studies trialling new technologies for COVID-19 tests.
“This just wouldn’t have been possible without that built-in research nurse structure.
“This is good for UK science, it’s good for investment, we already had the access to patients and we now have an infrastructure which can do a lot of the research delivery.
“But the key to delivering any study are the research nurses. If we want to further transform research delivery in this country, investing in research nurses is absolutely the way to do it.”
- To celebrate the work and impact of the NHS research workforce, the NIHR has been running its Shape The Future campaign throughout November – find out more here: https://www.nihr.ac.uk/explore-nihr/campaigns/nhs-75/

World-leading Quantitative Genetics Professor to share his insights in lunchtime lecture
One of the world’s leading statistical and quantitative genetics researchers will be talking about some of his work in a lunchtime lecture on 1 December at the Victor Phillip Dahdaleh Heart and Lung Research Institute.
Aimed at staff and researchers working on the Cambridge Biomedical Campus, Professor Peter Visscher’s talk will look at “Exploiting within-family segregation variance to study complex traits,” and show unpublished results from an analysis of more than 100,000 siblings pairs with GWAS and trait data.
Professor Visscher is known for his research investigating the genetic basis of complex human traits, including common diseases. His research focuses on the causes and consequences of human trait variations and he was one of the first to propose, advocate and show that genome and trait data can be used to predict individuals who are genetically at high risk of disease. The use of “polygenic risk scores” in health care is now being trialled worldwide.
The talk will take place from noon on Friday 1st December at Room R109/101, VCP Heart & Lunch Research Institute. No booking is required and a light lunch will be available afterwards.
- Professor Visscher is in the process of moving his Lab from the University of Queensland to the Big Data Institute, University of Oxford.
- If you’d like to meet with Professor Visscher, please contact Michael Inouye (mi336@cam.ac.uk mi336@cam.ac.uk) cc Nicole Staudt (ns639@medschl.cam.ac.uk ns639@medschl.cam.ac.uk), who are helping to coordinate his schedule.
Top research award for Neuroscience Theme co-lead Prof Hutchinson
Congratulations to our Neuroscience theme TBI (Traumatic Brain Injury) co-lead Professor Peter Hutchinson, who has been awarded the Vilhelm Magnus Medal from the Norwegian Neurosurgical Association in Oslo, following delivery of the prestigious Vilhelm Magnus Lecture.
The award, which is often described as the “Nobel Prize of Neurosurgery”, was given to Prof Hutchinson and his team for their work on TBI, including a number of studies addressing the concept of “Rescuing the Injured Brain.”
It is the latest in a long list of acclaimed work by Prof Hutchinson, who is also Director of the National Institute of Health and Care Research Global Health Research Group on Acquired Brain and Spine Injury, and the National Institute of Health and Care Research Brain Injury Medtech Co-operative.
The photo shows Prof Hutchinson (right) receiving the medal from Professor Tor Ingebrigtsen.
Watch Professor Hutchinson and Neuroscience Theme Lead Professor Chinnery talk about the theme’s research in this short video:
Shaping the future for women with prolapse
Cambridge physiotherapist Claire Brown believes a physiotherapist-led pessary service for women with prolapse could be life-changing – and has embarked on a year-long research fellowship as a first step to investigating this.
Pelvic floor dysfunction is a common and distressing condition which can affect all adults but particularly women who have had children.
Symptoms include bladder and bowel problems, pelvic organ prolapse (where organs slip down from their normal position into the vagina) and pain, affecting patients’ quality of life and limiting their life choices.
Claire, a clinical specialist pelvic health physiotherapist at Addenbrooke’s, said: “As a physio I work closely with my specialist network group, Pelvic Obstetric and Gynaecological Physiotherapy (POGP), and three years ago we worked as part of a multi-disciplinary team to launch clinical guidelines for best practice in using vaginal pessaries for prolapse.
“These are simple devices that you pop inside the vagina to support the internal walls and reduce the symptoms of a vaginal prolapse.

A vaginal pessary is a mechanical device (not a hormonal pessary) inserted inside a vagina to relieve prolapse symptoms. There are many different types, materials and sizes of pessaries. This is a ring pessary.
“But the guidelines were not backed up by sufficient evidence to show pessaries were effective in the younger age groups we wanted to target, which may explain why they haven’t been widely adopted.
“That’s incredibly frustrating for us as clinicians, and even more for the women with prolapses because this treatment could work.”
So Claire is now on a mission to collect the evidence needed to convince health practitioners to adopt clinical guidelines for vaginal pessaries for younger women; this autumn she started a full-time research fellowship, during which she will conduct qualitative interviews with women patients who have vaginal prolapse and with the GPs, nurses and physiotherapists who look after them.
The research is funded by Addenbrooke’s Charitable Trust and the Evelyn Trust, and facilitated through the NIHR Cambridge BRC’s Training and Professional Development team.
Claire said: “This will provide the pilot data that I need for my research, including finding out what makes access to treatment easier – and harder?
“Another key part of my research will be forming a PPI steering group, this will be invaluable because the direction we take will be steered by members’ direct experience of prolapse treatment – and how easy or difficult it was to access.
Getting the evidence
Claire wants to concentrate on collecting the data – or evidence – to show that pessaries can help women with prolapses.
She said: “The women who could benefit aren’t getting the treatments, partly because doctors aren’t offering pessaries to women because there is an age bias.
“Most of pessary research is conducted in the older population, however childbirth is one of the biggest risk factors for developing a prolapse.
“But clinically, pessaries work, but we need the hard evidence to back this up, and to show pessaries are acceptable to younger women as a treatment option.”
Claire has a few simple words of advice to others thinking about working in research: “Do it – because if you don’t follow your passions, you’ll regret it. Then keep going!”
What’s next for Claire?
“I plan to apply for the Doctoral Clinical and Practitioner Academic Fellowship (DCAF) scheme, which will enable me to undertake a PhD by research.
“This is funded by NHS England and NIHR, and if successful I will spend approximately 80% on my research and around 20% in clinical practice.
“Thanks to NIHR Cambridge BRC which enabled this research, I will have pilot data and more experience in conducting my own research to underpin my application.
“It’s another fantastic opportunity for me personally but even more, I hope, for the women who will benefit from service improvements as a result of the research.”
- This November NIHR is launching its Shape the Future autumn campaign, to celebrate the work and impact of the NHS research workforce. It encourages researchers to learn more about research, explore research careers and how to add research to their practice, learning and development.
- If you’re a healthcare proressional in the NHS and are inspired by Claire’s story and are interested in finding out more about pursuing a career in research, visit our Training & Professional Development area on our website for training opportunities and contacts.
November events for your diary: save the dates!
Want to find out more on how research improves patients’ outcomes? Or on the problems of the health and social care workforce from different research perspectives?
Two events taking place in November may be just the ticket.
NIHR Cambridge BRC public open evening: What difference does research make in improving hospital care?
It’s a fact – research-active hospitals have better patient outcomes. And in our first in-person public open evening since lockdown, you can find out more about some of our ground-breaking research in cancer, obesity, endocrine tumours and hearing loss.
Taking place at the Cancer Research UK Cambridge Institute on Tuesday 7th November, our researchers in will look at the difference research is making in improving hospital care.
You will have the opportunity to ask questions, and there will be time afterwards for networking. Refreshments will be available on arrival and after the lectures.
Doors open at 5.30pm, with the talks taking place from 6-7.30pm.
Register online for NIHR Cambridge BRC open evening
This event is suitable for members of the public and healthcare staff. Booking is essential. To find out more visit our Open Evening page on our website or go straight to EventBrite to book your ticket.
We need to talk about the workers: Researching the health and social care workforce: online lecture
The 2023 lecture from the Cambridge Centre for Health Services Research (CCHSR) will take place online on Tuesday, 14 November 2023, 12.30–1.30pm. Professor Jill Manthorpe CBE from King’s College London will explore the problems of the health and social care workforce from different research perspectives and how solutions seem to rise and fall in popularity.
Themes will include the UK reliance on international recruitment, numerous recruitment initiatives, scant retention interest, and a surprising short-sightedness in preparing for workforce ageing or workforce caring responsibilities. Research interest in the workforce is growing and ideas for approaches and studies will be canvassed.
About the speaker
Jill Manthorpe is Professor Emerita at King’s College London Policy Institute. For 20 years she was Director of the Policy Research Unit in Social Care Workforce and then the Policy Research Unit in Health and Care Workforce.
Register online
To book your free place for Researching the health and social care workforce: online lecture please visit EventBrite.
To book your free place for NIHR Cambridge BRC open evening please register on EventBrite.
Risks from smoking while pregnant more than double previous estimates
Cambridge researchers have shown that women who smoke during pregnancy are 2.6 times more likely to give birth prematurely compared to non-smokers – more than double the previous estimate.
The study analysed data collected during the Pregnancy Outcome Prediction (POP) study, which was supported by NIHR Cambridge BRC, and is published today in the International Journal of Epidemiology.
It also found that smoking meant that the baby was four times more likely to be small for its gestational age, putting it at risk of potentially serious complications including breathing difficulties and infections.
But the team found no evidence that caffeine intake was linked to adverse outcomes.
Women are currently recommended to stop smoking and limit their caffeine intake during pregnancy because of the risk of complications to the baby. For example, smoking during pregnancy is associated with an increased risk of fetal growth restriction, premature birth and low birthweight, though it has also been linked to a reduced risk of preeclampsia (high blood pressure during pregnancy).
High caffeine intake has also been shown to be associated with lower birthweights and possibly fetal growth restriction. Caffeine is more difficult to avoid than cigarette smoke as is found in coffee, tea, chocolate, energy drinks, soft drinks, and certain medications.
Studies looking at the links between smoking, caffeine and adverse pregnancy outcomes tend to rely on self-reported data to estimate exposure, which is not always reliable. A more objective measure is to look at levels of metabolites in the blood – chemical by-products created when substances such as tobacco and caffeine are processed in the body.
Researchers at the University of Cambridge and the Rosie Hospital, part of Cambridge University Hospitals NHS Foundation Trust, recruited more than 4,200 women who attended the hospital between 2008 and 2012 as part of the POP study. The team analysed blood samples taken from a subset of these women four times during their pregnancies.
To assess exposure to cigarette smoke, the team looked at levels of the metabolite cotinine, which can be detected in blood, urine, and saliva. Only two out of three women with detectable levels of cotinine in every blood sample were self-reported smokers, showing that this measure is a more objective way of assessing smoking behaviour.
A total of 914 women were included in the smoking analysis. Of these, 78.6% were classified as having no exposure to smoking while pregnant, 11.7% as having some exposure and 9.7% as having consistent exposure.
Compared to women who were not exposed to smoking while pregnant, those with consistent exposure were 2.6 times more likely to experience spontaneous preterm birth – more than double the previous estimate of 1.27 from a meta-analysis of studies – and 4.1 times as likely to experience fetal growth restriction.
Babies born to smokers were found to be on average 387g lighter than babies born to non-smokers – that is, more than 10% smaller than the weight of an average newborn. This increases the risk that the baby will have a low birth weight (2.5kg or less), which in turn is linked to an increased risk of developmental problems as well as poorer health in later life.
Unlike in previous studies, however, the team found no evidence that smoking reduced the risk of pre-eclampsia.
Professor Gordon Smith, Head of the Department of Obstetrics and Gynaecology at the University of Cambridge, said: “We’ve known for a long time that smoking during pregnancy is not good for the baby, but our study shows that it’s potentially much worse than previously thought. It puts the baby at risk of potentially serious complications from growing too slowly in the womb or from being born too soon.
“We hope this knowledge will help encourage pregnant mums and women planning pregnancy to access smoking-cessation services. Pregnancy is a key time when women quit and if they can remain tobacco free after the birth there are lifelong benefits for them and their child.”
Smoking cessation is offered routinely to all pregnant women and the NHS has local smoking cessation services for anyone, pregnant or not. Further information is available on the NHS website.
To assess caffeine intake, they researchers looked for the metabolite paraxanthine, which accounts for 80% of caffeine metabolism and is both less sensitive to recent intake and more stable throughout the day.
915 women were included in the caffeine analysis. Of these women, 12.8% had low levels of paraxanthine throughout pregnancy (suggesting low caffeine intake), 74.0% had moderate levels and 13.2% had high levels. There was little evidence of an association between caffeine intake and any of the adverse outcomes.
Professor Charlotte Coles receives top honour from Royal College of Radiologists
Congratulations to NIHR Cambridge BRC cancer researcher Professor Charlotte Coles, CRUK RadNet Cambridge lead, on being awarded the Gold Medal by the Royal College of Radiologists.
The Gold Medal is the highest honour that the College can give to a Fellow (radiologist or clinical oncologist) for important work that benefits patients.
Charlotte, who is Professor of Breast Cancer Clinical Oncology and NIHR Research Professor at the University of Cambridge, and Honorary Consultant in Clinical Oncology at Addenbrooke’s Hospital, leads practice-changing research on the best way to deliver radiotherapy treatment to breast cancer patients.
Her research aims to provide breast cancer patients with the best chance of cure with least side effects by personalising radiation techniques based on risk of recurrence.
Charlotte’s work has influenced international hypofractionation policy and she is Chair of the Lancet Breast Cancer Commission, an international multidisciplinary team aiming to influence global policy and improve the lives of people at risk of, and living with, breast cancer.
She leads CRUK RadNet Cambridge, one of seven centres of excellence across the UK pioneering new radiotherapy technologies and techniques to provide better radiotherapy treatments for patients with fewer side effects.
On receiving the Gold Medal at a ceremony at Central Hall Westminster last week, she said: “I feel very honoured and privileged to receive this award on behalf of collaborative patient-centred research in breast cancer and radiation therapy research.”
BRC-supported invention to be showcased at major industry fair
A digital health invention supported by NIHR Cambridge BRC is to be showcased at a major industry fair in London.
BloodCounts!, developed by NHS Blood and Transplant’s Dr Nicholas Gleadall and Dr Michael Roberts at the University of Cambridge, will be presented by NIHR Cambridge BRC partner Cambridge Enterprise at the IP4U University Tech Fair on 19-20 September.
It’s the first time that the Technology Transfer Offices (TTOs) of the University of Cambridge, Imperial College London, Oxford University and University College London have held such an event, which will showcase 80 inventions in sustainability and health.
IP4U will be an opportunity for industry to some of the researchers behind the innovations, and find out how they can partner with academics to commercialise their research.
Early warning system
BloodCounts! uses data from routine blood tests and powerful AI-based techniques to scan for abnormal changes in the blood cells of large populations. Based on this information, doctors can then alert public health agencies to potential emerging infectious disease outbreaks,
The development of the algorithms used in BloodCounts! was only possible due to the EpiCov data-sharing initiative pioneered by Cambridge University Hospitals (CUH) and funded by NIHR Cambridge BRC. The EpiCov database contains de-identified patient and NHS staff data from the CUH Electronic Health Record systems, including scan images and laboratory results.
It includes routinely collected information about patients diagnosed with COVID-19 or suspected of having COVID-19, and staff who have been tested for COVID-19. It also includes information about a large number of control patients who do not have a diagnosis of COVID-19.
- For more information including how to register visit https://ip4u.tech.
Prestigious laureate award for Theme Lead Prof Farooqi
Our Nutrition, Obesity, Metabolism and Endocrinology Theme Lead Professor Sadaf Farooqi has been given the prestigious 2024 Outstanding Clinical Investigator award from the Endocrine Society.
The award honours an internationally recognized clinical investigator who has contributed significantly to understanding the pathogenesis and therapy of endocrine and metabolic diseases. It is one of only a handful of Laureate Awards made by the society each year, to celebrate the achievements of the world’s top endocrinologists.
Professor Farooqi researches the fundamental mechanisms that control human energy homeostasis. She discovered the first genes whose disruption causes severe obesity and established that the principal driver of obesity is a failure of the central control of appetite. She also is a keen advocate to raise more awareness around weight stigma and obesity as a disease.
I am delighted and honoured to receive this prestigious award which recognises the dedication and contributions of past and present team members. I would particularly like to thank the many patients and volunteers who have contributed to our clinical research over the years, allowing us to find new ways to diagnose and treat people with severe obesity.
Professor Farooqi
The Endocrine Society is a global community of physicians and scientists, dedicated to accelerating scientific breakthroughs and improving patient health and well-being. Their main annual meeting, now called ENDO, has been held each year since 1916, except for 1943 and 1945 during World War II. Professor Farooqi will be presented with her award at ENDO 2024 in June next year.
