NIHR Cambridge BRC and CRF begin working on new COVID-19 study
Research staff from NIHR Cambridge Clinical Research Facility (CRF) are being deployed onto the wards at Cambridge University Hospitals (CUH) to start using a new test for a research trial into COVID-19.
COVIDx, a study supported by the NIHR Cambridge BRC, aims to investigate the impact of two new tests for COVID-19 on delivering faster diagnoses and understanding the development of immunity following infection. The first part of the study will evaluate the accuracy of the new SAMBA II-based test and whether it speeds up the diagnosis of COVID-19 in a ‘real-time’ hospital setting at the point of care.
The second part will investigate a point of care finger prick ‘antibody’ test of the blood, to determine how quickly markers of immunity appear following infection and a positive SAMBA test. These antibody tests will be important for understanding which patients and staff have already had the infection, and may be safe to return to work following recovery.
Research nurses detecting COVID-19
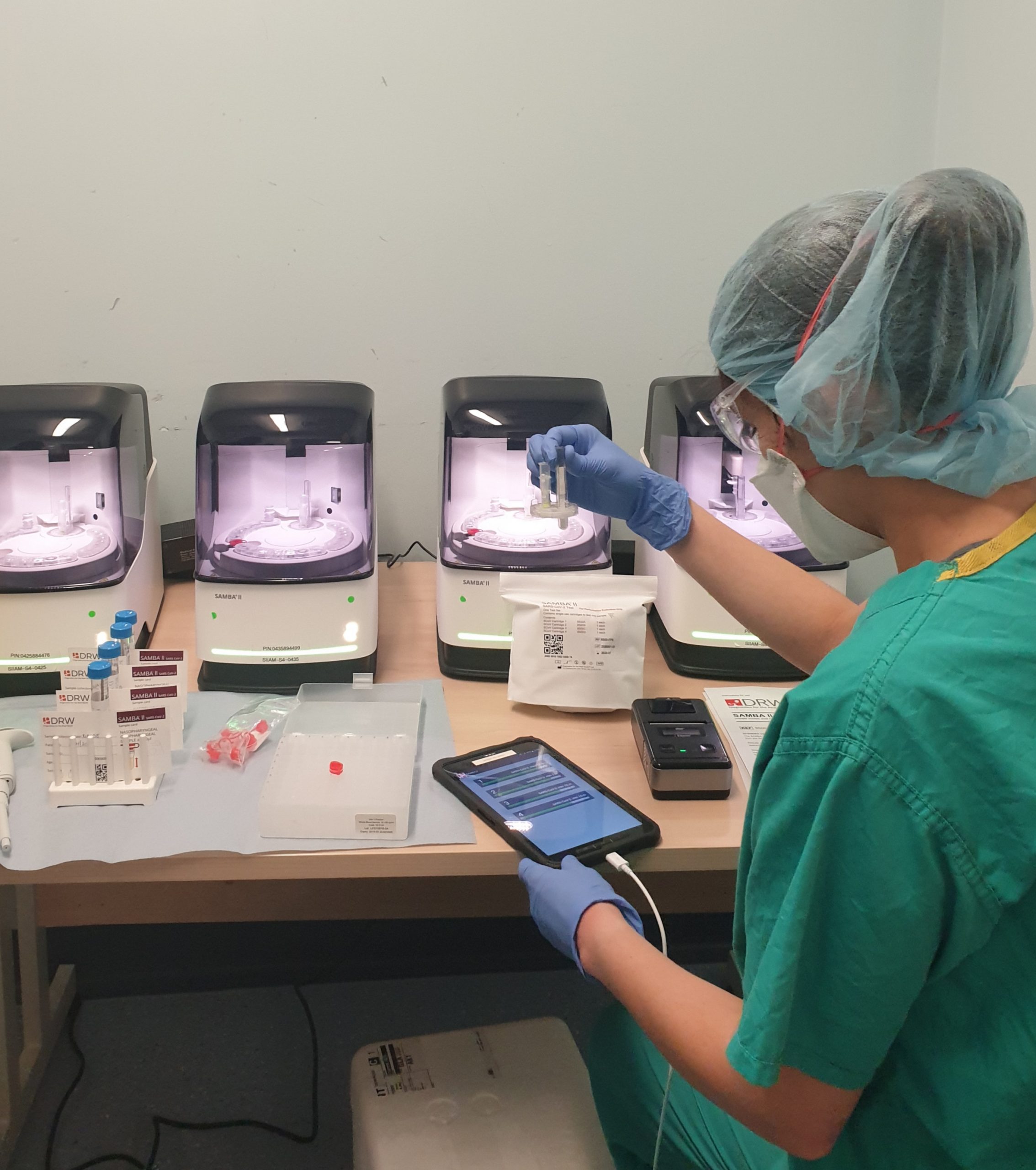
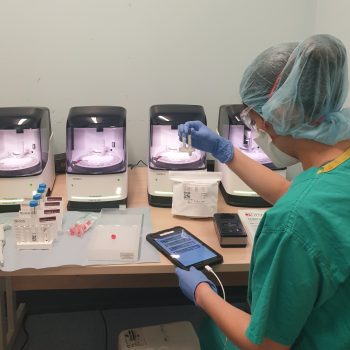
Research nurses working with the SAMBA II machines
Research nurses from the NIHR Cambridge CRF are collecting samples from patients with suspected COVID-19 to support the COVIDx study. Vivien Mendoza, NIHR Cambridge CRF lead research nurse said: “Our research nurses are working on a triage ward at CUH dedicated for patients who may have COVID-19.
“We are using the SAMBA II machine to test nasal and throat swabs to determine if a patient has COVID-19 and if the new device is an improved source of testing. The new test only takes 90 minutes and we will be matching these swabs against the results of standard practices.”
Currently, swabs taken from patients are sent off to the lab to be analysed, which can take a few days to return a result. The SAMBA II test can provide extremely reliable results in less than two hours, meaning decisions about clinical care or self-isolation can be made much more rapidly. The antibody tests require serum from blood samples, which will be tested in specialised facilities at the Cambridge Institute for Therapeutic Immunology and Infectious Diseases (CITIID).
Vivien added: “SAMBA II produces a summary of the test and the results are sent to a tablet and can be forwarded on to the clinician. When the clinicians receive the results they are able to start the correct care pathway for the patient a lot faster. We will also be testing NHS staff members who are working in COVID-19 wards and ITUs.”
The new test will help those who may have been infected and those who are displaying non-specific symptoms (such as from hay fever or the common cold) return to work. This will avoid healthy staff going into self-isolation, supporting the delivery of front-line care.
Caroline Saunders, Director of Clinical Operations for NIHR Cambridge CRF said: “Research is vital in finding new treatments and testing new devices, COVID-19 shows exactly what our staff are trained for. Our nurses have tremendous experience and expertise in trialling new devices and treatments across a wide range of specialties and age ranges and have the skills to adapt to these new challenges.”
Testing patients and staff
Patients who are admitted to Cambridge University Hospitals and have suspected COVID-19 will be given the opportunity to take part in the COVIDx research trial, providing swabs and blood samples. They will also be given the opportunity to enrol in other COVID-19 related trials such as the RECOVERY study, currently on going at the hospital.
Once the two diagnostic tests have been validated in patients with confirmed COVID-19, the study will enrol a second group of participants – healthcare workers. The SAMBA II test will be able to quickly identify staff who are positive for COVID-19, even if they have no symptoms, allowing them to self-isolate or access treatment if required. The antibody test will be able to identify staff who have already had the infection, which will support frontline NHS staff over the epidemic.
Professor Ravi Gupta from the Cambridge Institute for Therapeutic Immunology and Infectious Disease, who is leading the COVIDx study said: “Like all new medicines, new tests and devices need to be assessed for their strengths and weaknesses. COVIDx is a month-long trial at CUH which will give us an opportunity to evaluate two new forms of point of care testing for COVID-19 in ‘real life’.
“By comparing 200 patient samples to the new screening tool, we will be looking at whether the new tests are faster and are more reliable than the existing practices. If we can prove the new test can provide a diagnosis in a fraction of the current waiting time, then we are able to triage patients to the right part of the hospital earlier and start their care a lot sooner.
“We are beginning to test staff in high-risk areas to help reduce the risk of infection and stop staff having to go into self-isolation unnecessarily. It is really important we can ramp up tests for everyone to make sure we can minimise the risk of infection and have better patient outcomes.”
Further information on SAMBA II
The technology behind SAMBA II was developed while Dr Helen Lee was at Cambridge’s Department of Haematology. The development of the technology has been supported by Wellcome, the Children’s Investment Fund Foundation, the US National Institutes of Health and Cambridge Enterprise, among others. For more information see the University of Cambridge press release
The SAMBA II has been initially rolled out at CUH before being launched in hospitals nationwide to test patients, and staff working in high-risk areas or intensive care units.