MPhil in Translating Devices and Advanced Therapies Research
An exciting MPhil opportunity is available in our Devices and Advance Therapies theme to help develop your skills and knowledge. The MPhil will begin in 2025, applications close on 3 December 2024.
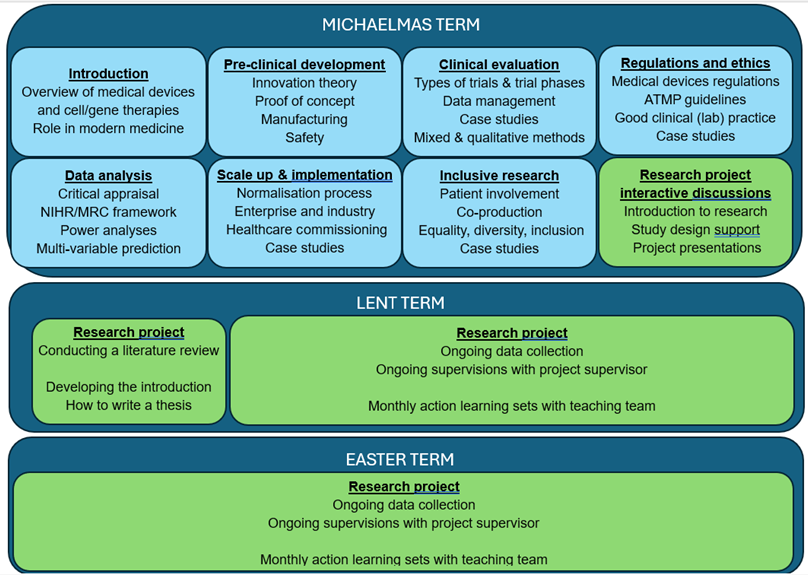
The Taught Course covers the following broad topics:
Pre-clinical development
- Innovation theory
- Proof of concept
- Manufacturing
- Safety
Clinical evaluation
- Types of trials and trial phases
- Data management
- Case studies
- Mixed and qualitative methods
Regulations and ethics
- Medical devices regulations
- AMP guidelines
- Good clinical (lab) practice
- Case studies
Data analysis
- Critical appraisal
- NIHR/MC framework
- Power analysis
- Multivariable prediction
Scale-Up and implementation
- Normalisation process
- Enterprise and industry
- Healthcare commissioning
- Case studies
Inclusive research
- Patient involvement
- Coproduction
- Equality, diversity, inclusion
- Case studies
Research project interactive discussions
- Introduction to research
- Study design support
- Project presentations
Brief overview and rationale for new course
The MPhil in ‘Translating Devices and Advanced Therapies Research’ is an exciting new course aimed at individuals who are developing or are interested in the development, obtaining regulatory approval and implementation of medical devices and/or cell and gene therapy interventions to improve patient outcomes.
This course aligns with the Cambridge Biomedical Research Centre (BRC) ‘Devices and Advanced Therapies’ theme.
Both the BRC theme and the MPhil are born out of a need expressed by researchers in clinical, academic and industry settings who do not have the skills and knowledge to develop and implement complex interventions.
The course also has an additional training option for those interested in becoming a ‘Qualified Person’ for advanced therapy interventions (the individual responsible for certifying the required standard and quality is met for cell and gene therapy for use in clinical trials).
Further information can be found on the University of Cambridge Postgraduate Study webpage or how to apply.
Contacts:
- James Tysome (Course Director)
- Matthew Smith (Course Director)
- Nick Haywood (Research Project Lead)