NIHR global health brain injury centre to open in Cambridge
A new research group to help improve the care of patients with traumatic brain injury in low and middle-income countries has been announced at the University of Cambridge and Addenbrooke’s Hospital.
Over 3,400 people die on the world’s roads every day and tens of millions of people are injured or disabled every year. Approximately half of these deaths are a result of traumatic brain injury (TBI). This is in addition to other causes, such as falls and violence. Over the past 20 years there have been major gains in the management of TBI in the UK and other high income countries (HICs) and there is now an urgency to address the increasing burden of TBI in low and middle income countries (LMICs).
The Department of Health has announced that 33 research units and groups will receive over £120 million of funding for Global Health Research after a successful open research competition led by the National Institute for Health Research (NIHR).
Among these, the Division of Neurosurgery at the University of Cambridge and Addenbrooke’s Hospital has been funded with £1.78 million to establish the NIHR Global Health Research Group on Neurotrauma. The Group will also benefit from the expertise of investigators from the Division of Anaesthesia, Institute of Public Health, and Department of Engineering. The Group will focus on interrogating the patient pathway and implementing strategies to improve the prevention, investigation, treatment and outcome of head-injured patients in LMICs.
The Group’s director, Peter Hutchinson – Professor of Neurosurgery at the University of Cambridge, said: “We are very excited for the opportunity to work with our colleagues and other partners in several LMICs in order to establish the Global Health Research Group on Neurotrauma. In addition, our strong partnerships with the World Federation of Neurosurgical Societies, the Society of British Neurological Surgeons, and the Headway charity, will allow us to further the Group’s objectives”.
The Group’s co-director, David Menon – Professor of Anaesthesia at the University of Cambridge, said: “The new Group will dovetail well with the ‘International Initiative for Traumatic Brain Injury Research’, an initiative where the Cambridge Acute Brain Injury programme already plays a leading role. We believe that knowledge exchange, in the course of the Group’s work, will benefit head-injured patients in LMICs but also HICs”.
The Group’s trainee co-lead, Angelos Kolias – Clinical Lecturer in Neurosurgery at the University of Cambridge, said:“A particularly important function of the Group will be the training of a cohort of young clinicians from the UK and the partner LMICs in global neurotrauma research, which has been made possible by this award. Developing the next generation of research leaders is critical for the Group’s long-lasting success”.
The formal launch of the NIHR Global Health Research Group on Neurotrauma will take place in September in Cambridge.
Written by Cambridge Neuroscience
Cambridge researcher tackles the health stories
NIHR Cambridge BRC researcher, Dr James Rudd, has been on secondment as one of 13 British Science Association Media Fellows at The Guardian.
In his day-to-day job James Rudd, cardiovascular researcher, works on finding new ways to predict heart attacks before they occur. However, he’s recently been given the opportunity to take on the science desk at The Guardian, shadowing science journalists as well as writing his own news stories on health and science.
A scheme running for 30 years, the Media Fellowship gives researchers a better understanding of how the media industry works. James has been learning what makes a good news story and getting used to tight deadlines that will all be beneficial to his research work. James has worked on several health stories such as peer support to breastfeeding mothers and the gonorrhoea superbug.
Read the full story by the British Heart Foundation and what James had to say about his time at The Guardian.
Concerns over side effects of statins stopping stroke survivors taking medication
Negative media coverage of the side effects associated with taking statins, and patients’ own experiences of taking the drugs, are among the reasons cited by stroke survivors and their carers for stopping taking potentially life-saving drugs, according to research published today.
Individuals who have had a stroke are at risk of a second stroke, which carries a greater risk of disability and death than first time strokes. In fact, one third of all strokes occur in individuals who have previously had a stroke. To prevent this recurrence, patients are offered secondary preventative medications; however, adherence is a problem with 30% of stroke patients failing to take their medications as prescribed.
To examine the barriers to taking these medications, researchers at the University of Cambridge and Queen Mary University, London (QMUL), analysed posts to TalkStroke, a UK-based online forum hosted by the Stroke Association, across a seven year period (2004-2011). The forum was used by stroke survivors and their carers.
The team, led by Dr Anna De Simoni, a lecturer in Primary Care Research at QMUL and visiting researcher at the Department of Public Health and Primary Care, University of Cambridge, has previously used the forum to explore issues such as the impairment that can make it difficult for stroke survivors to maintain a job.
The findings of the study, which looked at posts by 84 participants, including 49 stroke survivors and 33 caregivers, are published today in the journal BMJ Open. The Stroke Association gave the researchers permission to analyse the results, and to prevent identification of individuals, the team did not use verbatim comments.
Among the reasons cited by the forum users, side effects were a major factor in decisions to stop taking medication. Several contributors had experienced negative side effects and as a result had stopped taking the medication, sometimes in consultation with their GP and other times unilaterally. Others reported that they, or the person they were caring for, had stopped taking the medication after reading negative stories in the press about side effects.
Other users expressed concerns over the medication they were offered. There were conflicting views about the efficacy of the medications – some contributors believed they were very important, while others believed that their risk could be managed by lifestyle changes alone.
Contributors also reported mixed views of healthcare professionals – some felt confident in their doctor’s decision, while others questioned their decisions, some even questioning their motivation for prescribing particular drugs.
“These findings have highlighted the need for an open, honest dialogue between patients and/or their carers, and healthcare professionals,” says Dr De Simoni. “Doctors need to listen to these concerns, discuss the benefits and drawbacks of taking the medication, and be willing to support a patient’s informed decision to refuse medications.”
However, perceptions did not present the only barriers to adherence: there were often practical considerations. Drugs were sometimes too large and difficult to swallow, or a drug regime was too burdensome. The complexities of the drug regimens sometimes meant having to develop routines and strategies to ensure patients kept to them. One survivor described having to pay for the medications by credit card as she was unable to work and had no money or benefits coming in.
“By analysing people’s views as expressed in online forums, where they are more open and less guarded, we’ve seen some valuable insights into why some stroke survivors have difficulty adhering to their medication,” says PhD candidate and first author James Jamison from the Department of Public Health and Primary Care at Cambridge.
“Challenging negative beliefs about medication and adopting practices that make routines for taking medication simpler, particularly for those patients who have suffered disability as a result of stroke, should increase adherence and ultimately improve health outcomes.”
The research was supported by the National Institute of Health Research, the Stroke Association and the British Heart Foundation.
For more information about statins, visit NHS Choices.
Written by the University of Cambridge
‘Brain training’ app found to improve memory in people with mild cognitive impairment
A ‘brain training’ game developed by researchers at the University of Cambridge could help improve the memory of patients in the very earliest stages of dementia, suggests a study published today in The International Journal of Neuropsychopharmacology.
Amnestic mild cognitive impairment (aMCI) has been described as the transitional stage between ‘healthy ageing’ and dementia. It is characterised by day-to-day memory difficulties and problems of motivation. At present, there are no approved drug treatments for the cognitive impairments of patients affected by the condition.
Cognitive training has shown some benefits, such as speed of attentional processing, for patients with aMCI, but training packages are typically repetitive and boring, affecting patients’ motivation. To overcome this problem, researchers from the Departments of Psychiatry and Clinical Neurosciences and the Behavioural and Clinical Neuroscience Institute at the University of Cambridge developed ‘Game Show’, a memory game app, in collaboration with patients with aMCI, and tested its effects on cognition and motivation.
The researchers randomly assigned forty-two patients with amnestic MCI to either the cognitive training or control group. Participants in the cognitive training group played the memory game for a total of eight one-hour sessions over a four-week period; participants in the control group continued their clinic visits as usual.
In the game, which participants played on an iPad, the player takes part in a game show to win gold coins. In each round, they are challenged to associate different geometric patterns with different locations. Each correct answer allows the player to earn more coins. Rounds continue until completion or after six incorrect attempts are made. The better the player gets, the higher the number of geometric patterns presented – this helps tailor the difficulty of the game to the individual’s performance to keep them motivated and engaged. A game show host encourages the player to maintain and progress beyond their last played level.
The results showed that patients who played the game made around a third fewer errors, needed fewer trials and improved their memory score by around 40%, showing that they had correctly remembered the locations of more information at the first attempt on a test of episodic memory. Episodic memory is important for day-to-day activities and is used, for example, when remembering where we left our keys in the house or where we parked our car in a multi-story car park. Compared to the control group, the cognitive training group also retained more complex visual information after training.
In addition, participants in the cognitive training group indicated that they enjoyed playing the game and were motivated to continue playing across the eight hours of cognitive training. Their confidence and subjective memory also increased with gameplay. The researchers say that this demonstrates that games can help maximise engagement with cognitive training.
“Good brain health is as important as good physical health. There’s increasing evidence that brain training can be beneficial for boosting cognition and brain health, but it needs to be based on sound research and developed with patients,” says Professor Barbara Sahakian, co-inventor of the game: “It also need to be enjoyable enough to motivate users to keep to their programmes. Our game allowed us to individualise a patient’s cognitive training programme and make it fun and enjoyable for them to use.”
Dr George Savulich, the lead scientist on the study, adds: “Patients found the game interesting and engaging and felt motivated to keep training throughout the eight hours. We hope to extend these findings in future studies of healthy ageing and mild Alzheimer’s disease.”
The researchers hope to follow this published study up with a future large-scale study and to determine how long the cognitive improvements persist.
The design of ‘Game Show’ was based on published research from the Sahakian Laboratory at the University of Cambridge. The study was funded by Janssen Pharmaceuticals/J&J and Wellcome.
In 2015, Professor Sahakian and colleagues showed that another iPad game developed by her team was effective at improving the memory of patients with schizophrenia, helping them in their daily lives at work and living independently. The Wizard memory game is available through PEAK via the App Store and Google Play.
Reference
George Savulich, Thomas Piercy, Chris Fox, John Suckling, James Rowe, John O’Brien, Barbara Sahakian. Cognitive training using a novel memory game on an iPad in patients with amnestic mild cognitive impairment (aMCI). The International Journal of Neuropsychopharmacology; 3 July 2017; DOI: 10.1093/ijnp/pyx040
Written by the University of Cambridge
New research to help prevent older patients losing strength and function while in hospital
Research to find ways to improve frail older patients’ strength after a period in hospital will be carried out at the University of Cambridge by Addenbrooke’s Hospital physiotherapist, Peter Hartley, pictured.
After a period of hospitalisation, older people face substantial risks of leaving with new disabilities and dependencies that developed during their stay in hospital. Peter Hartley, Physiotherapy Team Lead in the Department of Medicine for the Elderly at Addenbrooke’s Hospital, will carry out a three year programme of research with older patients at Addenbrookes Hospital, thanks to his success in winning the highly competitive Dunhill Medical Trust Research Training Fellowship award.
The award will enable him to begin a PhD at the Department of Public Health and Primary Care at the University of Cambridge in October 2017. The research will focus on changes in muscle strength for frail older patients during their time in hospital and associated changes in patients’ mobility. Those most at risk of loss of muscle strength will be identified and an exercise intervention aimed at preventing loss of strength will be tested.
Professor Christi Deaton, Florence Nightingale Foundation Professor of Clinical Nursing Research, said: “Peter’s work has important implications for the care of hospitalised older patients. Preventing loss of muscle strength and functional decline could make a difference between a patient returning to independent living at discharge or needing greater support or even residential care. The Department of Medicine for the Elderly (DME) has goals of improving care and preventing harm to this vulnerable population, and Peter’s research will help to take this forward. We are very grateful to the support from DME and the Cambridge BRC/ACT internal research fellowship programme for supporting Peter in developing this competitive application.”
Cambridge researcher gets major award to take prostate biopsy device into clinical application
The CamProbe (Cambridge Prostate Biopsy Device), developed by Mr Vincent Gnanapragasam (University lecturer and Consultant Urologist at Cambridge University Hospitals) and his team, is a safer biopsy method for the early detection of prostate cancer, the most common cancer in men in the UK.
The current method of diagnosing prostate cancer is with a needle biopsy of the prostate guided by a transrectal ultrasound probe inserted into the rectum. This method and carries a significant risk of side effects including urinary infections and severe sepsis as the needle traverses the bowel a number of times on the way to the prostate.
The CamProbe will allow biopsies to be performed through the much more sterile transperineal route under local anaesthesia. In pilot trials the CamProbe resulted in no infections compared to rates of 5-12% from the current transrectal biopsy method. Moreover 8/10 men preferred the CamProbe approach over the current transrectal biopsy method and would recommend it to a relative or friend.
Mr Gnanapragasam, who co-leads the Urological Malignancies Programme at the CRUK Cambridge Centre said: “This is a fantastic opportunity to improve prostate cancer diagnotics and I am delighted that NIHR have chosen to invest in the CamProbe. Its use in hospital outpatient departments will mean a positive change in the experience of patients referred with suspected prostate cancer and a much safer way to diagnose the disease.”
This is the largest NIHR I4I award to be awarded to the University of Cambridge and only the second in a cancer theme. The i4i scheme funds the development of new healthcare technologies, devices and interventions for increased patient benefit in areas of existing or emerging clinical need.
Please note, this project is independent research funded by the National Institute for Health Research (Invention for Innovation (i4i) Programme grant II-LB-0716-20001). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, National Institute for Health Research or Department of Health.
Gateway to Genetic Counselling for Nurses and Midwives
Registrations now open and applications welcomed for assessed courses in Human Genomics & Genomic Counselling from the Cambridge Genomic Medicine Programme to prepare Nurses and Midwives for the increased role of genetics in healthcare.
• Funding available for NHS staff from Health Education England
• For information or to apply contact: Kath.Dogan@ice.cam.ac.uk
For information about this and other genomic medicine courses available to NHS staff in the East of England please contact Gemma Chandratillake Education & Training Lead for the East of England Genomic Medicine Centre: glb28@medschl.cam.ac.uk
Other modules are available through the Genomics Education Programme including postgraduate certificates in Genomic Medicine or Masters qualifications. Full details of courses taught throughout the UK available at Health Education England
Cambridge CRF nurses come out on top
Two of our NIHR Cambridge CRF nurses have won top prizes at a research conference in Cambridge. 
The Cambridge Research Conference is an annual event organised by Professor Christi Deaton to support the sharing of research projects and research training experiences/opportunities. The conference included a challenging 10-minute rapid fire context presentations and external speakers who are purposely invited by Prof Deaton in order to enrich researchers current knowledge.
The prize was a family ticket to Blenheim Palace, which was awarded to Antonella Ghezzi, NIHR CRF manager, (top right) for best poster – The NAFLD international project: a practice evaluation of health professionals’ experiences in the management of non-alcoholic fatty liver disease in the UK and India.
Jessica Taylor, NIHR CRF research sister (bottom right), presented a poster on Achieving Linchpin Effectiveness in Clinical Research Nursing Practice at the event. Jessica won a large box of biscuits as part of the research survey draw  .
.
Prof Deaton said: “It’s a fantastic opportunity to see the breadth of research undertaken by staff and how these are driven by questions from clinical practice. It is also wonderful to hear about the different options there are for increasing research knowledge and skills that are available locally and nationally, and how staff are successful in developing themselves.”
Cambridge celebrates International Clinical Trials Day
The NIHR BRC staff hosted a day of events on Thursday 18th May, to celebrate International Clinical Trials Day (ICTD) at Cambridge University Hospitals (CUH).
ICTD is part of a national campaign coordinated by the NIHR to commemorate the work of James Lind who pioneered clinical research with the first ever trial on the deadly disease scurvy.
In honour of the day, the NIHR Cambridge BRC held an information stand in the reception area of the outpatient’s department at CUH to promote clinical trials.
Research teams within the NIHR Cambridge BRC also took part in the event including, the Cambridge Clinical Trials Unit (CCTU), NIHR/Wellcome Trust Cambridge Clinical Research Facility (CRF), NIHR Cambridge BioResource and the Patient Led Research Hub (PLRH). They provided a range of information to the public about clinical trials and getting involved in research.
The NIHR Cambridge BRC spoke to people about signing up to the patient and public involvement panel. The panel invites members to volunteer in research by either taking part in focus groups, become a panel representative or review researcher’s documents from their home.
The CCTU ran a mock ‘chocolate trial’, and asked participants to rate their feelings after they ate some chocolate. The idea was for people to understand the process of consenting to a trial and how easy it is to take part in research.
Staff from the CRF talked about the kind of trials that take place there. They also wanted to understand the public’s knowledge of research within the hospital, and asked people to complete a survey on behalf of UK Clinical Research Facility Network.

Social media campaign
The NIHR Cambridge BioResource were looking for more volunteers to take part in research. In Cambridgeshire, they already have over 17,000 people – with and without health conditions who are available to take part in research. They talked to people about the process when you sign up to the NIHR Cambridge BioResource, and received enquiries from potential new members to join the panel.
Finally, the PLRH were on hand to talk about patients having the opportunity to propose their own research ideas to be taken forward and turned into clinical projects.
Throughout the day there was an on-going social media campaign, on the NIHR Cambridge BRC Twitter page titled #weareresearch #Iamresearch. Research staff took part holding up a sign with
their reasons of working in research and why it’s important. There were over 40 photos going out throughout the day with over 15,000 people seeing the tweets.
Thank you to everyone who took part in ICTD.
Results of public research awareness survey from CUH

Results from the public research awareness survey conducted at Cambridge University Hospitals (CUH) have now been released.
The survey was conducted in April until mid May 2017, and was led by the UKCRF Network PPI workstream. The survey ran in conjunction with the NIHR campaign ‘I Am Research’.
The survey team approached adults, family, staff and young people to understand their knowledge of research at CUH. Click on the poster to reveal the results.
NIHR Cambridge BRC Deputy Scientific Director is elected to Royal Society Fellowship
Krishna Chatterjee (Professor of Endocrinology), Deputy Scientific Director of the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (BRC), Director of the NIHR/ Wellcome Trust Cambridge Clinical Research Facility (CRF) and NIHR Senior Investigator, has been elected a Fellow of the Royal Society.
The Royal Society works to support outstanding contributions in science that benefit humanity. Fellowship members are selected for this prestigious honour, based on their own merit and ‘substantial contribution to the improvement of natural knowledge’. Professor Chatterjee’s fellowship award is based on his discoveries of genetic disorders of thyroid gland formation, hormone synthesis and hormone action, which have advanced fundamental knowledge of the thyroid axis.
On his newly elected status, Professor Chatterjee said: “I am honoured to be awarded a fellowship from the Royal Society which recognises the work of my research group, supported continuously by the Wellcome Trust, for over two decades. In addition, I would like to particularly acknowledge Clinical Research Infrastructure and Resources provided by the NIHR Cambridge BRC, which have enabled us to translate our fundamental discoveries into a national diagnostic service for unusual thyroid disorders and to trial new therapies for these disorders via our CRF.”
Rare “knockout” gene mutations in humans help scientists determine gene function
Identifying the function of every human gene is a key goal in medicine to understand health and what goes wrong in disease, but scientists still only know what a fraction of the estimated 20,000 genes in the human genome do.
In an international collaboration published in Nature, researchers from the University of Cambridge School of Clinical Medicine, Broad Institute of MIT and Harvard, University of Pennsylvania (UPenn), and the Center for Non-Communicable Diseases in Pakistan, analysed a Pakistani population in which relatives frequently intermarry to find humans with rare gene-disabling (“knockout”) DNA mutations and determine the consequences for health and disease.
In Pakistan, marriages between closely related individuals have been customary for generations, and children from such unions are more likely to inherit disabled versions of a gene from both parents, rendering them with no working copies of the gene. This study, with a cohort of more than 10,000 individuals, is one of the first to look in detail at such a population and the effects of these mutations. More than 1,300 genes were completely knocked out in at least one individual, allowing the research team to investigate what happens when there is complete loss of gene function.
The researchers showcase one particular knockout mutation in APOC3, a gene previously found responsible for maintaining dietary fat in the blood. The team identified a family with both copies of APOC3 disabled, and showed that these individuals are able to clear fat from their bloodstreams much more quickly than relatives who retained functional copies. When combined with an earlier report indicating that lacking one copy of APOC3 leads to lower risk for heart attack, these data suggest that a medicine developed to mimic these APOC3 knockout mutations could prevent heart attacks.
“The Human Genome Project revealed the genetic blueprint for human life, but we’re now entering a new era in genetics where we can systematically examine what it means for humans when parts of this blueprint are missing,” says co-senior author John Danesh, British Heart Foundation (BHF) Professor of Epidemiology and Medicine and Director of the MRC/BHF Cardiovascular Epidemiology Unit at Cambridge University and theme lead for the NIHR Cambridge BRC. “This has been made possible by advances in DNA sequencing technology and analytical capabilities to deal with the avalanche of data produced, combined with access to people who naturally lack functioning copies of particular genes.”
“To determine what a gene does, traditionally scientists ‘delete’ it in a mouse or a zebrafish and observe what happens. However, due to species differences, findings based on such experiments cannot always be extrapolated to humans,” says James Peters, BHF Clinical Research Fellow in Danesh’s group. “In contrast, this study leverages the high degree of consanguinity (relatedness) in Pakistan to find naturally-occurring gene knock-outs with direct relevance to human health and disease”.
Prof. Danesh, along with the paper’s first author Danish Saleheen, led the assembly of a cohort in Pakistan to study the genetic risk of heart disease. Participants provided blood samples for genetic sequencing and analysis of more than 200 biological traits such as cholesterol and insulin levels. But in the sequence data from the first few thousand participants, the researchers noted a large number of knocked-out genes, and pivoted to study the consequences of these genetic variations.
Previous human cohort studies have generated lists of potentially disabled genes in participants, but the team went a step further in the current study, correlating the nonfunctional genes with the physiological measurements. Within the cohort, the researchers found 1,317 genes where at least one person had both of their copies disabled. Roughly a third of these disabled genes existed in two or more people, allowing for further analysis, and the team determined that seven of the disrupted genes were associated with at least one of the measured traits.
Most notable was a family in which the parents, who were first cousins, both had two disabled copies of the gene APOC3. The research team returned to this family for clinical evaluation and found that those without a working version of APOC3 cleared dietary fat from their blood at a faster rate than other relatives could with one or two functional APOC3 genes. The natural power of APOC3-disabling mutations to lower this risk factor for heart disease could be a boon for preventative therapies, following the roadmap laid out by study of a different gene called PCSK9. “Studying individuals with gene knock-outs can help guide drug development, by mimicking the effects of a drug that blocks the protein made by that gene”, says Danesh. In 2005, a group of researchers reported that a nonfunctional version of PCSK9 was associated with lower blood cholesterol, and a year later, researchers described an adult woman with two non-functional copies of PCSK9 — indicating that humans could tolerate complete loss of this gene. Such observations eventually led to the development of medications that block PCSK9, and these medications are now approved by the U.S. Food and Drug Administration to lower blood LDL cholesterol.
“This research provides a framework that could be scaled up to establish a global ‘human knockout’ project: scouring populations for humans with naturally-occurring knockout mutations, and determining the range of physical consequences stemming from that gene-function loss to better understand the genome,” says co-first author Pradeep Natarajan, a postdoctoral research fellow in Sekar Kathiresan’s lab at the Broad Institute. Going forward, this study suggests that genome sequencing efforts focused on highly consanguinous populations will provide the greatest yields.
Written by the University of Cambridge, this research was funded by the NIHR Cambridge BRC
Improved imaging of vascular inflammation: CHAI and VISION studies
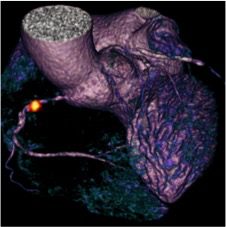
RCA imaged with DOTA
Dr James Rudd, Dr Elizabeth Warburton, Dr Anthony Davenport, and others have published two studies on the imaging of vascular inflammation.
The first study – CHAI – was funded by the British Heart Foundation and supported by the NIHR Cambridge BRC. CHAI study is the first prospective human study to quantify hypoxia in atherosclerosis using 18F-fluoromisonidazole (FMISO) PET. Symptomatic carotid plaques were found to be more hypoxic than asymptomatic lesions, potentially identifying a novel target for drug therapy. The robust correlation between 18F-fluorodeoxyglucose (FDG) and FMISO signals suggests that hypoxia contributes to the FDG signal in FDG PET studies of atherosclerosis.
The second study – VISION – was funded by the Wellcome Trust and supported by the NIHR Cambridge BRC. In the study, 68Ga-DOTATATE PET has been used successfully as a novel marker of atherosclerotic inflammation. Compared to FDG, 68Ga-DOTATATE offered superior coronary imaging, excellent macrophage specificity and better power to discriminate high-risk vs. low-risk lesions.
Both papers have been published in the same issue of JACC (Vascular imaging with 18F-fluorodeoxyglucose positron emission tomography is influenced by hypoxia; Detection of atherosclerotic  inflammation by 68Ga-DOTATATE PET compared to 18F-FDG PET imaging). The research is hoped to open up new therapeutic avenues for atherosclerosis, the leading cause of death in the UK
and worldwide and the driver of most heart attacks and strokes.
inflammation by 68Ga-DOTATATE PET compared to 18F-FDG PET imaging). The research is hoped to open up new therapeutic avenues for atherosclerosis, the leading cause of death in the UK
and worldwide and the driver of most heart attacks and strokes.
The studies reflect the hard work of many people, cutting across disciplines within the Clinical School and beyond. For his work on the VISION study, Dr Jason Tarkin (pictured 2nd from right) was awarded the Young Investigator award from the American Heart Association in November 2016. For his efforts in the CHAI study, Dr Francis Joshi was awarded an American Heart Association Early Career Investigator Award and a British Atherosclerosis Society Binks Trust Award.
Researchers shine at Cambridge Science Festival

Researchers from the National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre (BRC), took part in the Cambridge Science Festival on Sunday 26th March at the Cambridge Biomedical Campus. Hundreds of people visited the site and took a keen interest in the research happening on the campus.
As well as meeting the researchers, visitors had the opportunity to look at some of the equipment researchers use everyday, look at their current work they are doing on the campus and ask questions.
There were a wide range of displays to showcase the research and encourage children to become interested in science. With one of her real lab microscopes on display, Dr Romina Vuono talked to visitors about her Parkinson’s research, examples of her work and how the brain functions.
Teng and his team were there to talk to visitors about stroke research. Using water and ink, they had set up a circulation system to demonstrate what a healthy circulation system looks like and what we can do to maintain our own.
Crina a research associate demonstrated to children how immune cells worked by getting them to make their own bracelets. The Cambridge BRC Patient and Public Involvement team spoke to potential new members about what the panel does – reviewing research documents and working with researchers.
The Rare Diseases team talked about rare diseases and explaining how our DNA makes us all unique using the example of colouring in a large zebra.
The NIHR Cambridge BioResource were on hand to talk to people about the BioResource and how people can get involved in research, with the opportunity to sign up to the service. The team a played a game with children to understand their genetics.
From the 1st April 2017, the NIHR Cambridge BRC began its next phase with an £114m investment from the Government to make world-first breakthroughs for the next five years, looking into 17 research themes. Professor John Bradley, Director of the NIHR Cambridge Biomedical Research Centre, said on winning the award: “We are delighted that our research excellence has been recognised by this massive investment. We look forward to continuing our work to translate Cambridge’s outstanding biomedical research into benefits for patients.”
NIHR IBD BioResource celebrates 1000th patient
The National Institute of Health Research (NIHR) Inflammatory Bowel Disease (IBD) BioResource are celebrating the recruitment of their 1,000th patient to their research programme.
Inflammatory Bowel Disease (IBD) is a term used to describe two conditions, Crohn’s disease and Ulcerative Colitis. These lifelong illnesses flare at intervals, producing debilitating symptoms including cramping abdominal pains, anaemia, weight loss and diarrhoea. They require on-going drug therapy, and many patients also require major surgery.
In the UK, more than 300,000 people are affected by IBD. The exact causes of Crohn’s disease and Ulcerative Colitis are unclear, but there is evidence that IBD can cluster in families and having an affected family member is a risk factor.
In January 2016, a new research resource was launched nationally to recruit people with IBD to help investigators better understand Crohn’s and colitis and find new treatments. The NIHR IBD  BioResource sits within the NIHR BioResource – comprising volunteers from around the England who are willing to be approached to participate in research studies and trials on the basis of their genetic makeup or clinical features.
BioResource sits within the NIHR BioResource – comprising volunteers from around the England who are willing to be approached to participate in research studies and trials on the basis of their genetic makeup or clinical features.
The NIHR IBD BioResource began in Cambridge just over a year ago, and the team have had a lot of interest across the country from people who want to take part. More centres are opening up for recruitment. Cambridge has recruited its 1,000th patient, and nationally 1,000 more have been recruited in the last few months alone.
The IBD BioResource recruits people diagnosed with Crohn’s disease or Ulcerative Colitis and asks them to provide a small blood sample and clinical data. Researchers then study the genetic makeup of these samples. People who sign up to the IBD BioResource are then invited back to participate in future research studies and this could involve giving another blood sample or a stool sample, completing a questionnaire or participating in a trial.
Dr Miles Parkes, a consultant gastroenterologist at Cambridge University Hospitals and national lead for the NIHR IBD BioResource project, said: “We have been delighted by the positive response to this project since we launched it in January 2016. 1,000 of our Cambridge patients with Crohn’s or colitis are now signed up to help research into these conditions – and we hope that many more from around the UK will join in the months ahead. We want to get 25,000 recruits over the next 3 years. The whole point is to have an interactive resource which allows the amazing progress in genetics seen in recent years to be translated into clinical benefit.”
Wendy Edwards, Research Manager at Crohn’s and Colitis said: “The recruitment of the first 1000 patients to the IBD Bioresource is a landmark moment and we are delighted to have part funded the project. With access to this growing resource, researchers can identify new pathways to better diagnosis and treatments for Crohn’s Disease and Ulcerative Colitis. The IBD Bioresource offers new hope to people who live with these chronic conditions on a daily basis, by opening new doors to new discoveries.”
Researchers hope more people will come forward to sign up as volunteers for the NIHR IBD BioResource, to help better understand IBD and develop new treatments for those who are living with this condition.
More information can be found at: NIHR IBD BioResource or NIHR BioResource


 .
.



 inflammation by 68Ga-DOTATATE PET compared to 18F-FDG PET imaging
inflammation by 68Ga-DOTATATE PET compared to 18F-FDG PET imaging

 BioResource sits within the NIHR BioResource – comprising volunteers from around the England who are willing to be approached to participate in research studies and trials on the basis of their genetic makeup or clinical features.
BioResource sits within the NIHR BioResource – comprising volunteers from around the England who are willing to be approached to participate in research studies and trials on the basis of their genetic makeup or clinical features.