Drinking more than five pints a week could shorten your life, study finds
Regularly drinking more than the recommended UK guidelines for alcohol could take years off your life, according to new research from the University of Cambridge. Part-funded by the British Heart Foundation, the study shows that drinking more alcohol is associated with a higher risk of stroke, fatal aneurysm, heart failure and death.
The authors say their findings challenge the widely held belief that moderate drinking is beneficial to cardiovascular health, and support the UK’s recently lowered guidelines.
The study compared the health and drinking habits of over 600,000 people in 19 countries worldwide and controlled for age, smoking, history of diabetes, level of education and occupation.
The upper safe limit of drinking was about five drinks per week (100g of pure alcohol, 12.5 units or just over five pints of 4% ABV beer or five 175ml glasses of 13% ABV wine). However, drinking above this limit was linked with lower life expectancy. For example, having 10 or more drinks per week was linked with one to two years shorter life expectancy. Having 18 drinks or more per week was linked with four to five years shorter life expectancy.
The research, published today in the Lancet, supports the UK’s recently lowered guidelines, which since 2016 recommend both men and women should drink no more than 14 units of alcohol each week. This equates to around six pints of beer or six glasses of wine a week.
However, the worldwide study carries implications for countries across the world, where alcohol guidelines vary substantially.
The researchers also looked at the association between alcohol consumption and different types of cardiovascular disease. Alcohol consumption was associated with a higher risk of stroke, heart failure, fatal aortic aneurysms, fatal hypertensive disease and heart failure and there were no clear thresholds where drinking less did not have a benefit.
By contrast, alcohol consumption was associated with a slightly lower risk of non-fatal heart attacks.
The authors note that the different relationships between alcohol intake and various types of cardiovascular disease may relate to alcohol’s elevating effects on blood pressure and on factors related to elevated high-density lipoprotein cholesterol (HDL-C) (also known as ‘good’ cholesterol). They stress that the lower risk of non-fatal heart attack must be considered in the context of the increased risk of several other serious and often fatal cardiovascular diseases.
The study focused on current drinkers to reduce the risk of bias caused by those who abstain from alcohol due to poor health. However, the study used self-reported alcohol consumption and relied on observational data, so no firm conclusions can me made about cause and effect. The study did not look at the effect of alcohol consumption over the life-course or account for people who may have reduced their consumption due to health complications.
Dr Angela Wood, from the University of Cambridge, lead author of the study said: “If you already drink alcohol, drinking less may help you live longer and lower your risk of several cardiovascular conditions.
“Alcohol consumption is associated with a slightly lower risk of non-fatal heart attacks but this must be balanced against the higher risk associated with other serious – and potentially fatal – cardiovascular diseases.”
Victoria Taylor, Senior dietician at the British Heart Foundation, which part-funded the study, said: “This powerful study may make sobering reading for countries that have set their recommendations at higher levels than the UK, but this does seem to broadly reinforce government guidelines for the UK.
“This doesn’t mean we should rest on our laurels, many people in the UK regularly drink over what’s recommended. We should always remember that alcohol guidelines should act as a limit, not a target, and try to drink well below this threshold.”
The study was funded by the UK Medical Research Council, British Heart Foundation, National Institute for Health Research, European Union Framework 7, and European Research Council.
From the University of Cambridge, adapted from a press release by British Heart Foundation.
New gene discovery may help thousands with pulmonary arterial hypertension
Scientists say they have identified genes that cause a deadly heart condition – pulmonary arterial hypertension (PAH) – that can only be cured by transplants of the heart or lungs.
Pulmonary Arterial Hypertension (PAH) is a fatal lung disease and causes the walls of the arteries become thick and stiff, narrowing the space for blood to pass through and increasing blood pressure then leading to heart failure.
The disease kills 50% of those affected within five years, but little was known about what caused the condition in some people. Now experts say they have discovered five genes that cause the illness and could pave the way for more treatments.
Scientists carried out the largest ever genetic study of the disease by analysing the genomes – the unique sequence of a person’s DNA – of more than 1,000 PAH patients for whom the cause of the illness was unknown.
They found that mutations in five genes were responsible for causing the illness in these people, including in four genes that were not previously known to be involved in the disease. In people with the condition these genes fail to effectively produce the proteins that are required for the structure, function and regulation of the body’s tissues, researchers found.
Nick Morell, the lead author of the paper and professor at the NIHR Cambridge BRC and British Heart Foundation, said: “Identifying the nature of these new genes and mutations in the new genes tells you what causes the disease.
“It allows you to design and come up with potential new ways of treating the disease because you have really well-grounded knowledge about what’s actually causing it in cases where you find these mutations,” he explained.
More information about this research can be found in Nature Communications
The NIHR Cambridge Biomedical Research Centre, NIHR BioResource, BHF Cambridge Centre of Cardiovascular Research Excellence, the UK Medical Research Council supported this study
NIHR IBD BioResource reaches milestone of 10,000 recruits
A platform for research into Crohn’s Disease and Ulcerative Colitis has now signed up 10,000 participants nationwide.
The National Institute for Health Research (NIHR) IBD BioResource was established in 2016 by the UK IBD Genetics Consortium and the NIHR BioResource, to build on knowledge from recent genetics advances and accelerate the development of new treatments in Crohn’s disease and Ulcerative Colitis. Participants who have signed up to the NIHR IBD BioResource can be accessed by any investigators in the UK with an ethically approved research project, hence dramatically speeding up such research projects.
Inflammatory Bowel Disease (IBD) is a term used to describe two conditions, Crohn’s disease and Ulcerative Colitis. These lifelong illnesses mostly affect young adults and flare at intervals, producing debilitating symptoms including cramping abdominal pains, anemia, weight loss and diarrhoea. They require on-going drug therapy, and many patients also require major surgery. The exact causes of Crohn’s disease and Ulcerative Colitis are unclear, but the last 10 years has seen major progress in understanding the genetic contribution to these conditions.

NIHR IBD BioResource team
In the UK, more than 300,000 people are affected by Crohn’s disease or Ulcerative Colitis, and despite major advances in characterising the genetic and some of the environmental factors that predispose, much work remains to be done to fully understand IBD and develop better treatments.
Since its launch two years ago, the NIHR IBD BioResource has been rolled out to recruit in 62 hospital sites across the country. Earlier this week it hit the major milestone of 10,000 participants.
Dr Miles Parkes, a consultant gastroenterologist at Cambridge University Hospital and lead for NIHR IBD BioResource, said: “Getting to the 10,000 recruitment mark is a fantastic achievement and I am very grateful to all who have helped to make this possible.
“The IBD BioResource is a nationwide effort, recruiting people who have Crohn’s disease or Ulcerative Colitis specifically so that they can help researchers to better understand the causes of IBD and develop better treatments.”
People who sign up to the NIHR IBD BioResource provide a blood sample and complete a short health questionnaire. They will be contacted regarding future IBD research projects for which they meet inclusion criteria and given further information. They then decide study by study if they would like to participate or not. Participation could be to completing an online survey, provide a fresh blood sample or even participating in a trial of a new treatment.
Researchers from Cambridge, Edinburgh, London, Manchester and Oxford have already used the service to expedite their IBD research.
Dr Parkes added: “Since its launch we have been delighted by the level of enthusiasm shown by recruitment sites and patients alike, and particularly by the scale of interest from scientific community to use the NIHR IBD Bioresource. We are grateful for the continued support of our funding partners, clinicians and patients, without whom the success of the NIHR IBD BioResource would not be possible.
“Importantly our on-going large-scale recruitment of patients will allow us to fulfill our mission – to facilitate outstanding IBD research in the UK.” 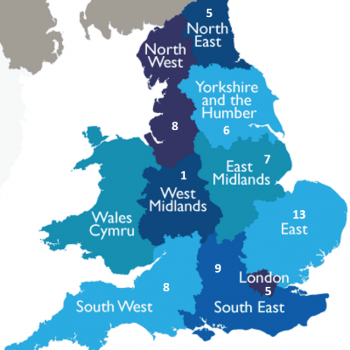
Helen Terry, Director of Research at Crohn’s and Colitis UK, said: “We are delighted to support this interactive BioResource which offers hope to thousands of patients suffering from Crohn’s Disease and Ulcerative Colitis (IBD).
“The 10,000 patients who have signed up are helping investigators to better understand and improve treatments for these debilitating, life-long illnesses. Access to this growing resource will enable researchers to translate the significant progress made in genetic research over the past few years into clinical benefit to improve the lives of people living with IBD and will open new doors to new discoveries.”
The NIHR IBD BioResource aims to open more sites across the country, with plans already for an extra 20 recruitment sites to be set-up by the autumn, with the long-term goal to achieve 25,000 participants.
For more information on the NIHR IBD BioResource, a website is available and explains how people with Crohn’s disease or Ulcerative Colitis can sign up, and how clinicians and investigators can get involved and access the NIHR IBD BioResource
Epic Research Seminar and Q&A session for all research staff
Following the high demand last year, the eHospital Research Design Authority has arranged another two Epic research seminars for research staff who actively use the Epic system at CUH.
These sessions will give research staff the opportunity to hear about the latest research specific developments and enhancements in the Epic system. There will be a presentation delivered by the eHospital Research team to demonstrate the features and functionality requirements, followed by an opportunity for staff to ask questions with the eHospital Research Design Authority team about using Epic for research studies. These seminars are open to all research staff at CUH who are active Epic users.
If you missed last year’s seminars, it is advisable for you to attend one of these sessions to understand the processes in the Epic system, meet and hear the latest information from the eHospital Research Design Authority and for you to feedback any issues. Places are limited and will be given on a first-come, first-served basis. The seminars will be on:
- 20 April, 09:00-12:00 – Room 4 Deakin Centre, Level 1
- 16 May, 14:00:17:00 – Room 4 Deakin Centre, Level 1
To book a place email: sylvie.robinson@addenbrookes.nhs.uk with ‘Seminar’ in the subject heading and with the date you would like to attend. You are invited to send any questions you have in advance of the seminar, so that the team have an opportunity to prepare an answer ahead of the event.
**This seminar is for all research staff at CUH who are active Epic users**
Extra EDGE training dates for Cambridge research staff
In March 2017, an announcement was made by the NIHR that instructed each Clinical Research Network (CRN) to obtain a Local Portfolio Management System on behalf of their partner organisations. The system chosen for use at Cambridge University Hospitals (CUH) by the CRN Eastern was EDGE.
Last year information was sent out to research staff to make sure they had nominated administrators to be trained and start recording study recruitment data on the system. Since then, research staff have been using this new cloud-based system to capture clinical trials data and support the delivery and maintenance of research at CUH.
EDGE is not a replacement for the Central Portfolio Management System (CPMS) but it does integrate into CPMS. EDGE will cover recruitment for CUH as a site but only for portfolio studies. Staff will still need to add recruitment data to CPMS for studies where CUH is the lead.
How to know if you need to receive the training?
- Do you have access to EPIC?
- Are you in a Research Team?
- Are you involved in an active Portfolio Research Study?
- Will you be responsible to upload recruitment for CUH?
If you have answered “YES” to all of these questions, you will need to attend the EDGE End User Training sessions. Choose the date below you would like attend and complete the form.
May
Wednesday 16th May 09:30 – 11:00
For more information email Paloma Amigo, LPMS Manager. Places are allocated on a first-come, first serve basis.
Analysis looks at long-term risks of living kidney donation
Living kidney donors are not at increased risk for some health outcomes previously of concern, but do seem at risk for worse blood pressure and kidney function than nondonors. In addition, female donors seem to be at increased risk for preeclampsia. The findings of a systematic review and meta-analysis are published in Annals of Internal Medicine.
A team lead by researchers from the University of Cambridge, England, reviewed 52 published studies comprising more than 100,000 living kidney donors and more than 110,000 nondonors to assess the mid- and long-term health risks associated with living kidney donation in adults.
The data showed that kidney donors had higher diastolic blood pressure, poorer renal function, and higher risk for ESRD than nondonors. Female donors had an almost two-fold higher risk than nondonors for pregnancy-related complications, such as preeclampsia.
There was no evidence that living kidney donors had higher risk for mortality, cardiovascular disease, or type 2 diabetes, or reduced quality of life. Lead author, Emanuele Di Angelantonio, MD, Director of the NIHR Blood and Transplant Unit (BTRU) in Donor Health and Genomics suggested that the findings may be used to inform prospective donors of the risks associated with kidney donation.
The authors of an editorial from the University of Pennsylvania write that despite 6 decades of living kidney donation, large and high-quality studies of ESRD and other relevant outcomes after donation have been completed only in the past decade. While the systematic review and meta-analysis provide some important answers, the field is still a long way from offering precise risk estimates to prospective donors.
The study was funded by the NIHR Blood and Transplant Research Unit with the NIHR Cambridge Biomedical Research Centre funding part of the study.
The Air We Breathe
Rajiv Chowdhury, University Lecturer in Global Health gave was interviewed on Cambridge TV to discuss the impact of air pollution. The interview was part of a programme to investigate the causes, affects and solutions of air pollution in Cambridge.
The piece aired on Sunday 21st January on Cambridge TV. If you missed it, click on the video below to catch up.
How incurable mitochondrial diseases strike previously unaffected families
Researchers have shown for the first time how children can inherit a severe – potentially fatal – mitochondrial disease from a healthy mother. The study, led by researchers from the MRC Mitochondrial Biology Unit at the University of Cambridge, reveals that healthy people harbour mutations in their mitochondrial DNA and explains how cases of severe mitochondrial disease can appear unexpectedly in previously unaffected families.
Mitochondrial diseases caused by mutations in mitochondrial DNA are rare, affecting approximately 1 in 10,000 births, but can cause severe conditions. For example, Leigh Syndrome is a severe brain disorder causing progressive loss of mental and movement abilities, which usually becomes apparent in the first year of life and typically results in death within two to three years.
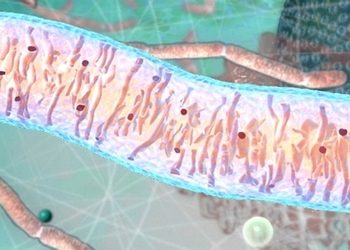
Mitochondria
Mitochondria are the powerhouses inside our cells, producing energy and carrying their own DNA instructions (separate from the DNA in the nucleus of every cell). Mitochondria are inherited from a person’s mother via the egg.
In the study, published in Nature Cell Biology, the researchers isolated mouse and human female embryonic germ cells – the cells that will go on to be egg cells in an adult woman – and tested their mitochondrial DNA.
They found that a variety of mutations were present in the mitochondrial DNA in the developing egg cells of all 12 of the human embryos studied, showing that low levels of mitochondrial DNA mutations are carried by healthy humans.
Professor Patrick Chinnery, from the MRC Mitochondrial Biology Unit and the Department of Clinical Neurosciences at the University of Cambridge, said: “We know that these devastating mitochondrial mutations can pop up in families without any previous history, but previously we didn’t know how that happened. We were surprised to find that egg cells in healthy females all carry a few defects in their mitochondrial DNA.”
For most of the human genome, mutations are kept in check by the processes of sexual reproduction, when eggs and sperm combine; however, mitochondria replicate asexually and mitochondrial DNA is inherited unchanged from the mother’s egg. This means that over time mutations can accumulate which, if left unchecked over generations, could eventually lead to malfunction and disease in offspring.
This conundrum led researchers to predict that a “bottleneck,” where only healthy mitochondria survive, may explain how mitochondria are kept healthy down the generations.
In this study, the researchers identified and measured this bottleneck for the first time in developing human egg cells. In these cells, the number of mitochondria decreased to approximately 100 mitochondria per cell, compared to around 100,000 mitochondria in a mature egg cell.
In a mature cell, a few faulty mitochondria could hide unnoticed amongst the thousands of healthy mitochondria, but the small number of mitochondria in the cell during the bottleneck means that the effects of faulty mitochondria are no longer masked.

Professor Patrick Chinnery
The exact mechanism by which cells with unhealthy mitochondria are eliminated is not yet known, but since developing egg cells need a lot of energy – produced by the mitochondria – the researchers suggest that after the bottleneck stage, eggs cells containing damaged mitochondria cannot generate enough energy to mature and are lost.
This study found every developing egg cell may carry a few faulty mitochondria, so occasionally, by chance, after the bottleneck these could be the mitochondria that repopulate the egg cell. The scientists suggest that if the quality-control step fails, then this faulty egg could survive and develop into a child with a mitochondrial disease.
Professor Patrick Chinnery said: “Unfortunately, the purification process is not perfect, and occasionally defective mitochondria leak through. This can cause a severe disease in a child, despite no one else in the family having been affected.”
Mitochondrial diseases are currently untreatable, although they can be prevented by the new technique of mitochondrial transfer – removing affected mitochondria from an egg or embryo and replacing them with healthy ones from a donor.
The study authors also suggest that this process could be relevant for human aging. Professor Chinnery added: “Previously it was assumed that the mitochondrial DNA mutations that have been associated with diseases of ageing, such as Alzheimer’s disease, Parkinson’s disease and other neurodegenerative disorders, happened over a person’s lifetime. This study shows how some of these mutations can be inherited from your mother, potentially predisposing you to late onset brain diseases.”
Professor Chinnery is a Wellcome Trust Senior Research Fellow and the researchers were funded by Wellcome, the Medical Research Council and the National Institute for Health Research.
Dr Nathan Richardson, MRC Head of Molecular and Cellular Medicine, said: “This is an exciting study that reveals important new insights into how mitochondrial diseases develop and are inherited between generations. The researchers have made great use of the tissues available from the MRC-Wellcome Human Developmental Biology Resource (HDBR). The HDBR is an internationally unique biobank resource that provides human embryonic and foetal tissue, donated through elective terminations, facilitating research into a large number of distressing medical disorders, such as mitochondrial diseases.”
The research is funded by the Wellcome Trust and the MRC with support from the NIHR Cambridge BRC.
Written by the MRC.
Advances in brain imaging settle debate over spread of key protein in Alzheimer’s
Recent advances in brain imaging have enabled scientists to show for the first time that a key protein which causes nerve cell death spreads throughout the brain in Alzheimer’s disease – and hence that blocking its spread may prevent the disease from taking hold.
An estimated 44 million people worldwide are living with Alzheimer’s disease, a disease whose symptoms include memory problems, changes in behaviour and progressive loss of independence. These symptoms are caused by the build-up in the brain of two abnormal proteins: amyloid beta and tau. It is thought that amyloid beta occurs first, encouraging the appearance and spread of tau – and it is this latter protein that destroys the nerve cells, eating away at our memories and cognitive functions.
Until a few years ago, it was only possible to look at the build-up of these proteins by examining the brains of Alzheimer’s patients who had died, post mortem. However, recent developments in positron emission tomography (PET) scanning have enabled scientists to begin imaging their build-up in patients who are still alive: a patient is injected with a radioactive ligand, a tracer molecule that binds to the target (tau) and can be detected using a PET scanner.
In a study published today in the journal Brain, a team led by scientists at the University of Cambridge describe using a combination of imaging techniques to examine how patterns of tau relate to the wiring of the brain in 17 patients with Alzheimer’s disease, compared to controls.
Quite how tau appears throughout the brain has been the subject of speculation among scientists. One hypothesis is that harmful tau starts in one place and then spreads to other regions, setting off a chain reaction. This idea – known as ‘transneuronal spread’ – is supported by studies in mice. When a mouse is injected with abnormal human tau, the protein spreads rapidly throughout the brain; however, this evidence is controversial as the amount of tau injected is much higher relative to brain size compared to levels of tau observed in human brains, and the protein spreads rapidly throughout a mouse’s brain whereas it spreads slowly throughout a human brain.
There are also two other competing hypotheses. The ‘metabolic vulnerability’ hypothesis says that tau is made locally in nerve cells, but that some regions have higher metabolic demands and hence are more vulnerable to the protein. In these cases tau is a marker of distress in cells.
The third hypothesis, ‘trophic support’, also suggests that some brain regions are more vulnerable than others, but that this is less to do with metabolic demand and more to do with a lack of nutrition to the region or with gene expression patterns.
Thanks to the developments in PET scanning, it is now possible to compare these hypotheses.
“Five years ago, this type of study would not have been possible, but thanks to recent advances in imaging, we can test which of these hypotheses best agrees with what we observe,” says Dr Thomas Cope from the Department of Clinical Neurosciences at the University of Cambridge, the study’s first author.
Dr Cope and colleagues looked at the functional connections within the brains of the Alzheimer’s patients – in other words, how their brains were wired up – and compared this against levels of tau. Their findings supported the idea of transneuronal spread, that tau starts in one place and spreads, but were counter to predictions from the other two hypotheses.
“If the idea of transneuronal spread is correct, then the areas of the brain that are most highly connected should have the largest build-up of tau and will pass it on to their connections. It’s the same as we might see in a flu epidemic, for example – the people with the largest networks are most likely to catch flu and then to pass it on to others. And this is exactly what we saw.”
Professor James Rowe, senior author on the study, adds: “In Alzheimer’s disease, the most common brain region for tau to first appear is the entorhinal cortex area, which is next to the hippocampus, the ‘memory region’. This is why the earliest symptoms in Alzheimer’s tend to be memory problems. But our study suggests that tau then spreads across the brain, infecting and destroying nerve cells as it goes, causing the patient’s symptoms to get progressively worse.”
Confirmation of the transneuronal spread hypothesis is important because it suggests that we might slow down or halt the progression of Alzheimer’s disease by developing drugs to stop tau from moving along neurons.
The same team also looked at 17 patients affected by another form of dementia, known as progressive supranuclear palsy (PSP), a rare condition that affects balance, vision and speech, but not memory. In PSP patients, tau tends to be found at the base of the brain rather than throughout. The researchers found that the pattern of tau build-up in these patients supported the second two hypotheses, metabolic vulnerability and trophic support, but not the idea that tau spreads across the brain.
The researchers also took patients at different stages of disease and looked at how tau build-up affected the connections in their brains.
In Alzheimer’s patients, they showed that as tau builds up and damages networks, the connections become more random, possibly explaining the confusion and muddled memories typical of such patients.
In PSP, the ‘highways’ that carry most information in healthy individuals receives the most damage, meaning that information needs to travel around the brain along a more indirect route. This may explain why, when asked a question, PSP patients may be slow to respond but will eventually arrive at the correct answer.
The study was funded by the NIHR Cambridge Biomedical Research Centre, the PSP Association, Wellcome, the Medical Research Council, the Patrick Berthoud Charitable Trust and the Association of British Neurologists.
Link to publication
Young people in research programme launches
In collaboration with the NIHR/Wellcome Trust Cambridge CRF and Trust’s Work Experience team, the NIHR Cambridge BRC Patient and Public Involvement (PPI) team have launched a Young People in Research Programme.
Twenty sixth-formers (from six local colleges) joined in October and have enjoyed a tour of the CRF with research sister Caroline McMahon as she gave an overview and tour of the facility.
The young people are now reviewing the new children’s pages for the CRF website and will be taking part in various patient and public involvement/engagement activities. They will be meeting researchers from a wide range of backgrounds and be introduced to staff representing the wider research team. There will also be a day of research inspired activities on 16th February here at the Cambridge Biomedical Campus for the group.
Some of our participants have a keen interest in the medical/ research field and could be future staff at the Cambridge Biomedical Research Campus. This great opportunity gives an insight into the world of health research, enabling young people to ask questions and to give us their opinions.
The programme runs until June 2018, for more information or to get involved, contact Anna Ellis PPI Coordinator or phone 01223 254620.
Research nurses present award winning work at international conference
The NIHR BioResource presented their award winning ‘Volunteer-Centric Model of Research Nursing’ at the International Association of Clinical Research Nurses.
The award-winning ‘Volunteer-Centric Model of Research Nursing’ published by the NIHR BioResource nursing team won a Nursing Times award in 2015.
NIHR BioResource research nurses Kelly Beer and Tracy Cook (pictured top left and centre) attended the conference in Rhode Island, USA to represent the NIHR BioResource team and present their model of research nursing. The focus for this year’s conference was “Clinical Research Nursing: Incorporating Professional Scope and Standards to Advance the Coordination and Care of Research Participants”.
Their presentation described the ‘Volunteer-Centric’ model, which facilitates the recruitment and engagement of volunteers in a translational research setting. The conference provided an excellent opportunity to showcase the team’s work and highlight the variety of research being undertaken at the Cambridge Biomedical Campus.
NIHR BioResource Research Nurse Tracy Cook said: “It was a fantastic opportunity to be able to present work that we are passionate about here at NIHR BioResource, and to share this with the wider research nurse community. The conference was inspiring and it is exciting to be part of an international group of research nurses. It highlighted that although we work in different countries with different healthcare cultures, we share the same challenges and are striving for the same outcomes.”
Lead Nurse Kelly Beer added: “We were very proud to be able to tell the audience about the amazing commitment and support that our volunteers give to the NIHR BioResource. It was a great pleasure to represent the NIHR BioResource at this event.”
Cambridge expert jointly leads international push to reduce global burden of traumatic brain injury
Substantial reductions in the global burden of traumatic brain injury (TBI) could be achieved with improved policies for prevention, new directions for clinical care, and novel approaches to research, according to The Lancet Neurology Commission on TBI.
The Commission has been co-led by Professor David Menon from the Division of Anaesthesia at the University of Cambridge and NIHR Cambridge BRC researcher, (pictured left), together with Professor Andrew Maas from Antwerp University Hospital and University of Antwerp, Belgium.
The Commission is being launched today at the European Parliament and targets policy makers, funders, and patient organisations, as well as health-care professionals. It combines the expertise of over 300 international clinicians and researchers, many of whom are part of the International Initiative for Traumatic Brain Injury Research (InTBIR). The authors set out clinical and research priorities with 12 key messages and recommendations to reduce the global burden of TBI.
The enormous and rising health and socioeconomic burden posed by TBI demands urgent action from health-care professionals and policy makers. About half the world’s population will suffer a TBI over their lifetime. TBI is estimated to affect 50 million people every year. It is the leading cause of mortality in young adults and a major cause of disability across all ages. It also substantially increases the risk of late-life dementia.
The care and consequences of TBI cost the global economy US$400 billion annually. Given an estimated gross world product of about $74 trillion, this means that about $1 in every $200 of annual global output is spent on the costs or consequences of TBI.
“Traumatic brain injury affects huge numbers of people worldwide, with potentially serious consequences for their health and wellbeing and a major economic burden on already stretched health services,” says Professor Menon. “We are not doing enough to prevent and manage such injuries, which is why we calling on policymakers, funders and healthcare professionals to take action.”
Increasing industrialisation and motor vehicle use are causing increases in TBI due to traffic incidents in low-income and middle-income countries, which disproportionately affect the young. In
high-income countries, incidence of TBI is highest and increasing in the elderly due to falls. Expectations of unfavourable outcomes in the elderly can lead to treatments being withheld or  prematurely withdrawn, with resulting poorer outcomes reinforcing therapeutic nihilism in the management of these patients. However, with appropriate care good results can be obtained.
prematurely withdrawn, with resulting poorer outcomes reinforcing therapeutic nihilism in the management of these patients. However, with appropriate care good results can be obtained.
More recently, substantial interest has focused on the health impact of sports-related concussion and its long-term effects. It is now recognised that repetitive injuries carry increased risks and that TBI should not be seen as an event, but as a process, often with lifelong consequences.
The Commission reports that understanding of TBI and care of patients is hampered by inconsistent epidemiological data, poor integration of systems of care, and substantial disparities in access to care. Furthermore, current medical management is inappropriately based on a one-size-fits-all approach. Inadequate attention to the condition’s heterogeneity at presentation and outcome might also be a substantial contributor to the failure of clinical trials of promising new therapies. Crucially, even when additional evidence is generated to improve management, the integration of such evidence into clinical guidelines and routine clinical care is slow. Most importantly, many cases of TBI are preventable, but well recognised measures to prevent the disease are not universally mandated in law or they are poorly implemented in practice.
The authors set out priorities and recommendations to address the varied challenges in understanding, prevention, and care of TBI, and seek to identify strategies to better characterise TBI, increase prognostic accuracy, and match treatments to patients—a precision-medicine approach.
The Commission also promotes use of new tools for clinical evidence generation and implementation, so that research outputs are more generalisable and can be more rapidly integrated into clinical care. Moreover, it highlights the importance of international collaboration of funding agencies and researchers to provide a global response to reduce the individual and societal burden of TBI.
Adapted by the University of Cambridge from a press release from The Lancet
Running on autopilot: scientists find important new role for ‘daydreaming’ network
A brain network previously associated with daydreaming has been found to play an important role in allowing us to perform tasks on autopilot. Scientists at the University of Cambridge showed that far from being just ‘background activity’, the so-called ‘default mode network’ may be essential to helping us perform routine tasks.
When we are performing tasks, specific regions of the brain become more active – for example, if we are moving, the motor cortex is engaged, while if we are looking at a picture, the visual cortex will be active. But what happens when we are apparently doing nothing?
In 2001, scientists at the Washington University School of Medicine found that a collection of brain regions appeared to be more active during such states of rest. This network was named the ‘default mode network’ (DMN). While it has since been linked to, among other things, daydreaming, thinking about the past, planning for the future, and creativity, its precise function is unclear.
Abnormal activity in the DMN has been linked to an array of disorders including Alzheimer’s disease, schizophrenia, attention-deficit/hyperactivity disorder (ADHD) and disorders of consciousness. However, scientists have been unable to show a definitive role in human cognition.
Now, in research published today in the Proceedings of National Academy of Sciences, scientists at the University of Cambridge have shown that the DMN plays an important role in allowing us to switch to ‘autopilot’ once we are familiar with a task.
In the study, 28 volunteers took part in a task while lying inside a magnetic resonance imaging (MRI) scanner. Functional MRI (fMRI) measures changes in brain oxygen levels as a proxy for neural activity.
In the task, participants were shown four cards and asked to match a target card (for example, two red diamonds) to one of these cards. There were three possible rules – matching by colour, shape or number. Volunteers were not told the rule, but rather had to work it out for themselves through trial and error.
The most interesting differences in brain activity occurred when comparing the two stages of the task – acquisition (where the participants were learning the rules by trial and error) and application (where the participants had learned the rule and were now applying it). During the acquisition stage, the dorsal attention network, which has been associated with the processing of attention-demanding information, was more active. However, in the application stage, where participants utilised learned rules from memory, the DMN was more active.
Crucially, during the application stage, the stronger the relationship between activity in the DMN and in regions of the brain associated with memory, such as the hippocampus, the faster and more accurately the volunteer was able to perform the task. This suggested that during the application stage, the participants could efficiently respond to the task using the rule from memory.
“Rather than waiting passively for things to happen to us, we are constantly trying to predict the environment around us,” says Dr Deniz Vatansever, who carried out the study as part of his PhD at the University of Cambridge and who is now based at the University of York.
“Our evidence suggests it is the default mode network that enables us do this. It is essentially like an autopilot that helps us make fast decisions when we know what the rules of the environment are. So for example, when you’re driving to work in the morning along a familiar route, the default mode network will be active, enabling us to perform our task without having to invest lots of time and energy into every decision.”
and energy into every decision.”
“The old way of interpreting what’s happening in these tasks was that because we know the rules, we can daydream about what we’re going to have for dinner later and the DMN kicks in,” adds senior author Dr Emmanuel Stamatakis from the Division of Anaesthesia at the University Of Cambridge. “In fact, we showed that the DMN is not a bystander in these tasks: it plays an integral role in helping us perform them.”
This new study supports an idea expounded upon by Daniel Kahneman, Nobel Memorial Prize in Economics laureate 2002, in his book Thinking, Fast and Slow, that there are two systems that help us make decisions: a rational system that helps us reach calculated decisions, and a fast system that allows us to make intuitive decisions – the new research suggests this latter system may be linked with the DMN.
The researchers believe their findings have relevance to brain injury, particularly following traumatic brain injury, where problems with memory and impulsivity can substantially compromise social reintegration. They say the findings may also have relevance for mental health disorders, such as addiction, depression and obsessive compulsive disorder, where particular thought patterns drive repeated behaviours, and the mechanisms of anaesthetic agents and other drugs on the brain.
This research was carried out in the general context of understanding conscious processing in the human brain. The Division of Anaesthesia, headed by Professor David Menon, NIHR Senior Investigator, has a programme of research aiming to further elucidate the neural basis of consciousness and cognition in health and disease.
The research was supported by the Yousef Jameel Academic Program, The Stephen Erskine Fellowship from Queens’ College Cambridge, and the NIHR Cambridge Biomedical Resource Centre.
Restless legs syndrome study identifies 13 new genetic risk variants
A new study into the genetics underlying restless legs syndrome has identified 13 previously-unknown genetic risk variants, while helping inform potential new treatment options for the condition.
As many as one in ten people of European ancestry is affected by restless legs syndrome, in which sufferers feel an overwhelming urge to move, often in conjunction with unpleasant sensations, usually in the legs. Rest and inactivity provoke the symptoms, whereas movement can lead to temporary relief. The condition is chronic and can get progressively worse, with long-lasting effects on patients’ mental and physical health. People with restless legs syndrome have substantially impaired sleep, reduced overall quality of life, and increased risk of depression, anxiety disorders, hypertension, and, possibly, cardiovascular disease.
For around one in 50 people, the condition can be severe enough to require chronic medication, which may in turn have potentially serious side effects.
Studies of families and twins have shown that there is a strong genetic component to the disorder and led to the discovery of six genetic variants that increased the risk of developing the condition.
“We have studied the genetics of restless legs syndrome for more than 10 years and the current study is the largest conducted so far,” says Dr Barbara Schormair from the Institute of Neurogenomics at the Helmholtz Zentrum München, leading first author of the study. “We are convinced that the newly discovered risk loci will contribute substantially to our understanding of the causal biology of the disease.”
Now, an international team of researchers has compared the genetic data from over 15,000 patients with more than 95,000 controls, and identified a further 13 genetic risk variants. The findings were then replicated in a sample of 31,000 patients and 287,000 controls. The results are published today in Lancet Neurology.
“Restless legs syndrome is surprisingly common, but despite this, we know little about what causes it – and hence how to treat it,” says Dr Steven Bell from the Department of Public Health and Primary Care at the University of Cambridge, also one of the first authors on the study. “We already know that it has a strong genetic link, and this was something we wanted to explore in more detail.”
Several of the genetic variants have previously been linked to the growth and development of nerve cells – a process known as neurogenesis – and to changes in the formation of neuronal circuits. These findings strengthen the case for restless legs syndrome being a neurodevelopmental disorder whose origins may go back to development in the womb as well as impaired nerve cell growth in later life.
“The genetic risk variants that we’ve discovered add more weight to the idea that this condition is related to the development of our nervous system,” says Dr Emanuele Di Angelantonio, also from the Department of Public Health and Primary Care. “It also gives us some clues to how we may treat patients affected by the condition.”
Prof Juliane Winkelmann, who heads the Institute of Neurogenomics at the Helmholtz Zentrum as well as a restless legs syndrome outpatient clinic at the Klinikum Rechts der Isar in Munich, adds: “Our genetic findings are an important step towards developing new and improved treatment options for our patients.”
One particular biological pathway implicated by the findings is known to be a target for the drug thalidomide. While the drug has a controversial reputation due to its previous use when treating pregnant women that led to serious birth defects in their offspring, it is now used to treat some cancers. The researchers suggest that thalidomide or similar drugs may offer potential treatment options for male patients with restless leg syndrome and female patients beyond reproductive age, but they stress the necessity of rigorous clinical testing for efficacy and side-effects before any such use.
The study was largely funded by NHS Blood and Transplant, National Institute for Health Research, British Heart Foundation, European Commission, European Research Council, National Institute for Health Research Cambridge Biomedical Research Centre, UK Medical Research Council, Deutsche Forschungsgemeinschaft (DFG) and Helmholtz Zentrum München.
Blood donors could give blood more often
New research shows that blood donors could safely give blood more frequently than is allowed at present. At the moment in the UK men can give blood every 12 weeks, women every 16 weeks. The new research may lead to the official recommendations being changed and the intervals between donations shortened.
Blood donations began more than a century ago but until now there has been no research to discover how frequently donors can give blood without it affecting their health.
The study showed that, over a two-year period, allowing donors to give blood more frequently boosted the supply of blood to the NHS without having a major impact on their health.
The study, published in the Lancet, was carried out by a team from Cambridge and Oxford Universities and NHS Blood and Transplant.
Supported by the UK Medical Research Council , British Heart Foundation and NIHR Cambridge Biomedical Research Centre the study involved 45,000 blood donors. The men were randomly assigned to groups giving blood at 8-, 10- and 12-week intervals; the women to groups giving blood at 12-, 14- or 16-week intervals. The results showed that, giving blood at the shorter intervals resulted in much more blood being collected without it having a major impact on the donors’ quality of life, mental function or physical activity. However, some of those who gave blood more frequently did report minor symptoms including tiredness and restless legs, and the research suggests this may have been due to giving blood.
According to the lead author, Dr Emanuele Di Angelantonio from the University of Cambridge, the study also showed that donors who weighed above average and those with higher initial stores of iron were able to give more blood.
Senior author Professor John Danesh, also from the University of Cambridge, said, “Our data give blood services the short-term option of more frequent collection from donors if the supply falls or demand rises. We have also measured how much iron is lost after two years of repeated donation, and this will help towards framing the safety guidelines in countries where blood donation is more frequent than in the UK.”
The results from the trial suggest that better screening methods should be sought to detect low haemoglobin in potential donors. NHS Blood and Transplant and the University of Cambridge have started the COMPARE study to test different ways to measure when a donor is anaemic and should not give blood.
According to another senior author, Professor Dave Roberts from the Univesity of Oxford, ‘In the future we can use our results to predict which donation intervals suit individual donors and move towards a more personalized donation interval.’
One of the donors who took part in the study, Paul Harvey, comes from a family of blood donors and both he and his brother were delighted to sign up.
‘I was on a 10 week cycle and the specialist team who looked after us made it easy to book my appointments for my next donation. Being able to give blood is a great way of giving something back to society and it is nice to know we might be able to do it more frequently as a result of this study”
Gail Miflin, NHS Blood and Transplants Medical and Research Director commented that “The INTERVAL study has shown how effectively we can collaborate with leading academics to generate the evidence to support our clinical practices. Our collection teams have been able to run donor recruitment and donation at different intervals within their normal working environment.” She added that “We now need to review the findings in greater depth to understand which donors can safely donate more frequently without this having an adverse impact on their iron stores. The study clearly shows that some donors could donate more frequently than the current donation intervals but it also highlights that some donors should donate at longer intervals.”
Genome editing reveals role of gene important for human embryo development
Researchers have used genome editing technology to reveal the role of a key gene in human embryos in the first few days of development in a study supported by NIHR Cambridge BRC. This is the first time that genome editing has been used to study gene function in human embryos, which could help scientists to better understand the biology of our early development.
The team used genome editing techniques to stop a key gene from producing a protein called OCT4, which normally becomes active in the first few days of human embryo development. After the egg is fertilised, it divides until at about 7 days it forms a ball of around 200 cells called the ‘blastocyst’. The study found that human embryos need OCT4 to correctly form a blastocyst.
“We were surprised to see just how crucial this gene is for human embryo development, but we need to continue our work to confirm its role” says Dr Norah Fogarty from the Francis Crick Institute, first author of the study. “Other research methods, including studies in mice, suggested a later and more focused role for OCT4, so our results highlight the need for human embryo research.”
Dr Kathy Niakan from the Francis Crick Institute, who led the research, adds: “One way to find out what a gene does in the developing embryo is to see what happens when it isn’t working. Now we have demonstrated an efficient way of doing this, we hope that other scientists will use it to find out the roles of other genes. If we knew the key genes that embryos need to develop successfully, we could improve IVF treatments and understand some causes of pregnancy failure. It may take many years to achieve such an understanding, our study is just the first step.”

Edited embryo
The research was published in Nature and led by scientists at the Francis Crick Institute, in collaboration with colleagues at Cambridge University, Oxford University, the Wellcome Trust Sanger Institute, Seoul National University and Bourn Hall Clinic. It was chiefly funded by the UK Medical Research Council, Wellcome and Cancer Research UK.
The team spent over a year optimising their techniques using mouse embryos and human embryonic stem cells before starting work on human embryos. To inactivate OCT4, they used an editing technique called CRISPR/Cas9 to change the DNA of 41 human embryos. After seven days, embryo development was stopped and the embryos were analysed.
The embryos used in the study were donated by couples who had undergone IVF treatment, with frozen embryos remaining in storage; the majority were donated by couples who had completed their family, and wanted their surplus embryos to be used for research. The study was done under a research licence and strict regulatory oversight from the Human Fertilisation and Embryology Authority (HFEA), the UK Government’s independent regulator overseeing infertility treatment and research.
As well as human embryo development, OCT4 is thought to be important in stem cell biology. ‘Pluripotent’ stem cells can become any other type of cell, and they can be derived from embryos or created from adult cells such as skin cells. Human embryonic stem cells are taken from a part of the developing embryo that has high levels of OCT4. “We have the technology to create and use pluripotent stem cells, which is undoubtedly a fantastic achievement, but we still don’t understand exactly how these cells work,” explains Dr James Turner, co-author of the study from the Francis Crick Institute. “Learning more about how different genes cause cells to become and remain pluripotent will help us to produce and use stem cells more reliably.”
Sir Paul Nurse, Director of the Francis Crick Institute, says: “This is exciting and important research. The study has been carried out with full regulatory oversight and offers new knowledge of the biological processes at work in the first five or six days of a human embryo’s healthy development. Kathy Niakan and colleagues are providing new understanding of the genes responsible for a crucial change when groups of cells in the very early embryo first become organised and set on different paths of development. The processes at work in these embryonic cells will be of interest in many areas of stem cell biology and medicine.”
Dr. Kay Elder, study co-author from the Bourn Hall Clinic, says: “Successful IVF treatment is crucially dependent on culture systems that provide an optimal environment for healthy embryo development. Many embryos arrest in culture, or fail to continue developing after implantation; this research will significantly help treatment for infertile couples, by helping us to identify the factors that are essential for ensuring that human embryos can develop into healthy babies.”
Dr Ludovic Vallier, study co-author from the Wellcome Trust Sanger Institute and the Wellcome – MRC Cambridge Stem Cell Institute, says: “This study represents an important step in understanding human embryonic development. The acquisition of this knowledge will be essential to develop new treatments against developmental disorders and could also help understand adult diseases such as diabetes that may originate during the early stage of life. Thus, this research will open new fields of opportunity for basic and translational applications.”
The research was funded by the Francis Crick Institute, which receives its core funding from Cancer Research UK, the UK Medical Research Council and the Wellcome Trust. With support from the NIHR Cambridge BRC.
Written and photos by Francis Crick
The fight to beat Alzheimer’s disease
As we live longer our chances of being diagnosed with a brain conditions such as Alzheimer’s disease increases. September the 21st is World Alzheimer’s day, and Cambridge researchers are taking the opportunity to highlight their research into this growing problem.
Dementia is an umbrella term for several different brain disorders which affect memory, thinking, behaviour and the ability to function in day-to-day tasks, of which Alzheimer’s disease is the most prevalent. There are many types of dementia including vascular dementia, Lewy body and other parkinsonian dementias and Frontal lobe dementia. Common to many, is that the cause of the disease is an increase in certain proteins, including amyloid and tau in Alzheimer’s disease, and alpha-synuclein in Lewy body dementias. In the UK 850,000 people are estimated to be living with dementia.

Professor John O’Brien
Professor of Old Age Psychiatry, John O’Brien, from the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (BRC) is leading a team of researchers working to better understand the causes of dementia, and to find new treatments. “We still don’t fully understand why the disease occurs, though we know it develops over many years and is more common in older people, in those with certain genes and in those with some lifestyle factors. Not smoking, making sure your blood pressure and weight is controlled, having a healthy diet, exercise and keeping your mind active are all positive things to keep you healthy. However, age is a big risk factor, so just because you’re fit and healthy doesn’t mean you won’t develop dementia. That’s why we are doing more research to understand why it develops.”
Dementia symptoms affect a person’s daily life; in particular Alzheimer’s disease can cause confusion, memory loss or difficulties with speech. John explains, “It’s unlikely we will find a cure, but what we hope to see in the next few years are better symptomatic drugs, which is something we’re working on to reduce the effects of the disease and give people a better quality of life. Also new drugs that will start to slow the progression of the disease, which can be given early in the illness to help preserve independence.”
Researchers at the NIHR Cambridge BRC are working alongside clinicians at Cambridge University Hospitals to investigate why the disease occurs. “There’s a lot of research happening in Cambridge to understand dementia overall,” explains John. “We have groups looking at the basic chemical changes of the proteins in the brain, cell cultures, stem cells, brain imaging, therapies and genetics.
“Some of our researchers are looking at the brain’s structure and how it functions, and whether changes such as inflammation in the brain are important. We’re lucky in Cambridge to have access to state-of-the-art brain imaging equipment to see the proteins in the brain so we can better understand why and when such changes happen, and what their effects are. We’re also looking at repurposing drugs – licensed drugs already in use for other diseases that might also be effective for people with dementia. Some of our researchers are conducting a prevention study – PREVENT Dementia – to monitor younger people and see if there are any indicators to those who may develop the disease later in life, so we will be able to detect it earlier.”
Dementia is a huge economic and social problem costing the economy £26 billion a year and the Government have invested more money into the UK to fund dementia research, though still a small proportion compared to equally important conditions like cancer. “It’s important we find new treatments as the cost of care is escalating.
proportion compared to equally important conditions like cancer. “It’s important we find new treatments as the cost of care is escalating.
“We have tremendous support from our patients, most of whom want to take part in research. They often report benefits from taking part and have said how this helps them feel empowered. They hope it can help future patients, and some benefits of taking part include the fact they will be amongst the first to have access to new treatments as part of clinical trials, and in the world of brain imaging, access to some of the latest scanning technologies. We work closely with Join Dementia Research, a national NIHR supported register which is able to support our research.”
Being properly assessed for any memory problems or symptoms that may be due to dementia is important, as memory problems can often be due to other medical illnesses or things like depression or anxiety. “Seeing your GP is vital if you’re worried about yourself or a relative. They will be able to assess for some causes of memory problems, and may refer you on to a specialist or memory clinic for further assessment. If the cause is dementia, then early diagnosis offers the best opportunity to benefit from current treatments and services for longer, as well as the opportunity to take part in research studies,” explains John.
Cambridge is one of the leading centres in England investigating new treatments for dementia, including Alzheimer’s disease. “We are really lucky here in Cambridge, the NIHR have been a fantastic support to let us conduct our work. Within the NIHR Cambridge BRC we have world renowned experts, first class infrastructure and excellent facilities – including the latest scanning equipment,” explains John. “Having World Alzheimer’s day is really important, it highlights this type of dementia and our work. But we also need to be mindful; as we are living longer it’s not just our physical selves that we need to look after but one of our most powerful organs– the human brain.”
More information of NIHR Cambridge BRC dementia research can be found here. Further information about the NIHR and dementia research can be found here.
Information about the Cambridgeshire and Peterborough NHS Foundation Trust can be found here
Older transplant kidneys could be given a new lease of life
Researchers from the NIHR Cambridge Biomedical Research Centre are investigating whether performing biopsies of older kidneys can help transplant patients and reduce waiting lists.
Kidney Research UK reports that in the UK there are approximately 5,200 people waiting for a kidney transplant, with only 3,300 transplants carried out each year. There are many reasons why people have kidney failure and find themselves on a donor waiting list. However, to find a suitably-matched kidney can normally take more than three years from the deceased-donor pool, and in some cases a match is never found.
One way to possibly increase kidney transplant numbers in the UK is to use more kidneys from older donors (over 60 years). Many more potential older donors die in intensive care units in the UK than younger donors, but their kidneys are often not used because kidney function declines as we get older, and because of the potential associated health problems that have developed in older patients.
The National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (BRC) is leading a new trial in the UK to determine whether performing an urgent biopsy (taking a small sample of tissue) on kidneys from older donors, to test their quality, can help to increase the number of deceased kidneys transplanted in the UK. 
As we age, our kidneys develop age-related chronic injury. The extent of this injury varies between individuals, but the biopsy will enable the severity of the injury to be assessed. The team will investigate whether the information obtained from the biopsy can be used to more effectively select those kidneys that are likely to provide better outcomes for transplantation. In so doing, it is anticipated that this will lead to an overall increase in the number of kidney transplants performed each year.
Mr Gavin Pettigrew, Chief Investigator from the NIHR Cambridge BRC and consultant surgeon explained: “A lot of older people who die in intensive care are potential kidney donors, but their kidneys are frequently not transplanted because of concerns that they may not function very well. We hope that the implementation of a dedicated National Histopathology Service will increase the numbers of kidneys transplanted in the UK each year, and that the outcome of these transplants will be favourable.”
To enable urgent biopsies to be performed on kidneys from donors in any hospital in the UK, the team have developed a system where the biopsy samples will be sent to one of six coordinating centres involved in this trial; Birmingham, Cambridge, Edinburgh, Leeds, London and Newcastle where special ‘digital’ scanners have been installed.
A biomedical scientist will prepare the biopsy, take a digital image and upload the sample onto a secure digital network that the histopathologist can access, even at home. The trial has assembled a team of dedicated histopathologists based at different hospitals throughout the UK who will provide their expert opinion. “By processing the biopsy while the kidney is in transit to the recipient, the pathologist will be able to give their opinion on the biopsy to the transplant team without prolonging the time that the kidney is stored on ice.

Mr Gavin Pettigrew
“This new kind of analysing a kidney could show kidneys from older donors are suitable for transplantation,”
Gavin explained. “We currently know there are a large number of kidneys from potential elderly deceased donors that are not used. We anticipate the biopsy service will enable kidneys from this pool to be used successfully, resulting in more kidney transplants being performed. This would then have the potential to save time and money and importantly patients’ lives who are urgently waiting for a kidney transplant.”
Dialysis can cost around £20,000 per patient per year, and puts a significant burden on a patient’s life. Being able to receive an older but healthy kidney would make a huge difference to those individuals and their families.
Elaine Davies Director of Research Operations at Kidney Research UK said: “With so few kidneys available for transplant, it would be a shame to reject a kidney unnecessarily when it could potentially give the precious gift of life. We welcome the news of this new trial to make older kidneys more viable, in the hope that this will increase the number of transplants carried out each year.”
The three year trial is due to begin in October, and has been funded by the NIHR. Cambridge University Hospitals and the University of Cambridge are sponsoring this trial with support from the NIHR Cambridge BRC. It will be managed by the NHS Blood and Transplant Clinical Trials Unit.
The team are currently setting up the six biopsy scanning centres, alongside 21 participating transplant centres participating in this trial in the UK. Gavin added: “Our end goal is that if this biopsy method works, we believe we can then perform an extra 100-150 kidney transplants a year. We would ideally like to roll it out to the other transplant centres and add more scanners across the UK thereby reducing the travel time.
“We could also potentially move this method into other areas such as liver, lung transplants and even cancer patients who need treatment. The hope of this new trial is not only to save time and money, but to save lives. We want more people to receive a healthy kidney faster so they can go back to leading a normal life.”
‘Fat but fit’ are at increased risk of heart disease
Carrying extra pounds could raise your risk of heart attack by more than a quarter, even if you are otherwise healthy.
Researchers have found that being overweight or obese increases a person’s risk of coronary heart disease (CHD) by up to 28% compared to those with a healthy bodyweight, even if they have healthy blood pressure, blood sugar and cholesterol levels.
The findings add to a growing body of evidence which suggests being ‘fat but fit’ is a myth, and that people should aim to maintain a body weight within a healthy range and shed the excess pounds.
Storing too much fat in the body is associated with a number of metabolic changes, including increased blood pressure, high blood sugar and lowering ‘good’ cholesterol levels, which can lead to disease and poor health.
However, previous studies have revealed a subset of overweight people who lack the ill health effects related to excess weight, leading to them being classified as ‘metabolically healthy obese’ in the medical literature, and ‘fat but fit’ in the media.
Now, a group led by researchers at Imperial College London and the University of Cambridge has shown that despite an apparent clean bill of health, this overweight group is still at increased risk compared to those with a healthy weight. In the largest study of its kind to date, scientists used data from more than half a million people in 10 European countries – taken from the European Prospective Investigation into Cancer and Nutrition (EPIC) – to show that excess weight is linked with an increased risk of heart disease, even when people have a healthy metabolic profile.
“Our findings suggest that if a patient is overweight or obese, all efforts should be made to help them get back to a healthy weight, regardless of other factors. Even if their blood pressure, blood sugar and cholesterol appear within the normal range, excess weight is harmful in itself” said lead author Dr Camille Lassale, from Imperial’s School of Public Health and now based at University College London.
In the study, published in the European Heart Journal, researchers looked at the link between excess weight and risk of CHD, a condition where not enough blood gets through to the heart due to clogged arteries, leading to heart attacks and heart failure.
After a follow-up period of more than 12 years, a total of 7,637 people in the EPIC cohort had experience CHD events, such as death from heart attack. Researchers then selected a representative random subcohort of more than 10,000 individuals as controls, for a case cohort analysis. All participants were grouped by health status and weight.
Body weight was classified according to definitions from the World Health Organization. Those with a body mass index (BMI) over 30 were classed as obese, while those with a BMI of 25–30 were classed as overweight, and 18.5–25 as normal weight. More than half of the subcohort (63%) were female, with an average age of 53.6 and an average BMI of 26.1.
Participants were categorised as ‘unhealthy’ if they had three or more of a number of metabolic markers, including high blood pressure, blood glucose, or triglyceride levels, low levels of HDL ‘good’ cholesterol, or a waist size of more than 37” (94cm) for men and 31” (80cm) for women.
After adjusting for lifestyle factors such as smoking, diet, exercise and socioeconomic status, the researchers found that compared to the healthy normal weight group, those classed as unhealthy had more than double the risk of CHD: with obese and unhealthy at 2.5 times the risk of CHD, overweight and unhealthy at 2.3, and unhealthy normal weight at 2.15 times the risk.
However, analysis also revealed that within the healthy group there was a significant difference in outcomes for people depending on their weight. The research found that compared to those at normal weight, people who were classified as healthy but overweight had an increased CHD risk of 1.26 (26%), while those who were healthy but obese had an increased risk of 1.28 (28%).
Dr Ioanna Tzoulaki, from Imperial’s School of Public Health, said: “I think there is no longer this concept of healthy obese. If anything, our study shows that people with excess weight who might be classed as ‘healthy’ haven’t yet developed an unhealthy metabolic profile. That comes later in the timeline, then they have an event, such as a heart attack.”
According to the researchers, the excess weight itself may not be increasing the risk of heart disease directly, but rather indirectly through mechanisms such as increased blood pressure and high glucose. They add that as no follow up measurements were taken, they cannot show how the group’s health status changed over time. However, they add that what is clear from the study is that population-wide prevention and treatment of obesity is needed in order to ensure public health.
Dr Lassale added: “Overall, our findings challenge the concept of the ‘healthy obese’. The research shows that those overweight individuals who appear to be otherwise healthy are still at increased risk of heart disease.”
The research was funded by the European Research Council and supported by the UK Medical Research Council and the British Heart Foundation and the National Institute of Health Research Cambridge Biomedical Research Centre.
Written by Imperial College London.
Cambridge welcomes next generation of scientists
 On Thursday 3rd August, we welcomed the London International Youth Science Forum. Entering its 59th edition, the two week trip for young scientists aged between 16-21 from over 70 different countries, got to visit some of the world leading research centres in the UK, with Cambridge being one of the main attractions.
On Thursday 3rd August, we welcomed the London International Youth Science Forum. Entering its 59th edition, the two week trip for young scientists aged between 16-21 from over 70 different countries, got to visit some of the world leading research centres in the UK, with Cambridge being one of the main attractions.
Around 50 people attended the visit to Cambridge University Hospitals (CUH) to come and meet researchers here on the Cambridge Biomedical Campus. They were given a talk on the campus’ rich history and proud achievements and heard talks from researchers.
Groups were then taken round some of the research facilities on the campus, such as the Cancer Research UK Cambridge Institute, Cambridge Institute for Medical Research, Histopathology/Tissue Bank, Institute of Metabolic Science, NIHR/ Wellcome Trust Cambridge Clinical Research Facility and the Wolfson Brain Imaging Centre.

Elizabeth (centre) with Ricardo (left) and Hugo (right)
Another attendee to the Cambridge event was Elizabeth Harvey. Elizabeth was on the 1997 visit and came to CUH as part of her tour. Elizabeth now works at CUH in anaesthetics. She kindly brought her booklet she was given at the 1997 event for the visitors and staff to see how much things have changed since then.
