Making chemotherapy for Hodgkin lymphoma kinder to patients
A simple change to the chemotherapy regimen for people with Hodgkin lymphoma could reduce the long-term health impacts that can result from treatment, according to researchers in Cambridge. The findings could lead to the national guidance on chemotherapy treatment for these patients being revised.
The study published in The Lancet Oncology was led by Cambridge University Hospitals, the Wellcome Sanger Institute and supported by the NIHR Cambridge BRC. It compares the lasting effects of two chemotherapy regimens used to treat Hodgkin lymphoma in younger adults. Hodgkin lymphoma is often diagnosed in younger people (age 20-40) so kinder treatments have the potential to deliver significant benefits, such as reduced hospital time and greater likelihood of recovering fertility.
Data previously collected from 1,945 patients treated with the existing chemotherapy regime (eBEACOPP) was compared to 312 patients treated with a similar regimen, called eBEACOPDac. Both treatments use combinations of drugs, and the change replaces one of these, procarbazine, with another called dacarbazine. Both chemotherapies achieved the same success in treating cancer (93.3% in remission 3 years after treatment), but comparison of data from the two groups showed that patients treated with eBEACOPDac generally experienced fewer, less severe side effects.
A similar drug substitution is already approved for use in children and is increasingly widely used for the treatment of adults, but this is the first study to rigorously examine its impact in adult patients.
Patients treated with the new regimen spent less time in hospital, required fewer blood transfusions following treatment, and more patients showed signs of recovering fertility sooner. This also has the potential to reduce hospital admissions and demand for hospital appointments. Part of the study used whole genome sequencing at the Wellcome Sanger Institute to look at the effects of both treatments and showed that eBEACOPDac has a greatly reduced impact on patient genes.
Hodgkin lymphoma is a rare, treatable blood cancer. Around 2,000 people per year are diagnosed in the UK and treatment success is high, with over 95% of younger patients cured with treatment. The occurrence of Hodgkin lymphoma in younger people means there is a significant need to reduce the long-term health and fertility impacts of treatment.
Chemotherapy is a well-established approach for treating various cancers, including Hodgkin lymphoma. There are many different chemotherapies consisting of several different drugs used in combination. Chemotherapies are highly effective for treating cancer but also have well-known side effects (e.g. nausea and hair loss) and can have lasting effects following treatment, including anaemia and infertility.
A commonly used standard first-line treatment for people with advanced Hodgkin lymphoma involves chemotherapy known as eBEACOPP. The new eBEACOPDac regimen does not increase the cost of treatment and is administered to patients in the same way. Making eBEACOPDac the recommended treatment for Hodgkin lymphoma in adults could improve the long-term health of patients and enable more of them to go on to have children.
Professor George Follows, Consultant Haematologist at Addenbrooke’s Hospital and the Department of Haematology, University of Cambridge and co-lead author on the study, said: “Our findings highlight the potential to make the short and long-term side effects of chemotherapy much kinder for Hodgkin lymphoma patients without compromising the effectiveness of treatment. By making a small change to how patients are managed, we can greatly reduce the lasting impacts that this disease, and its treatment, has on their lives giving many more patients the opportunity to go on to raise families.”
Dr Raheleh Rahbari, Wellcome Sanger Institute and co-lead author on the study, said: “This is an example of how genomics can impact lives and help change healthcare. Through the use of genome sequencing we’ve gained a deeper insight into the lasting effects of chemotherapies, allowing us to learn more about their role in long-term health, and make progress towards effective treatments that minimise side effects as much as possible.”
Dr Cathy Burton, Chair of the UK Hodgkin lymphoma study group, and Haematology Consultant at Leeds Teaching Hospitals NHS Trust, said: “This excellent work provides strong evidence of the benefits of using eBEACOPDac for treatment of Hodgkin lymphoma. This approach of switching procarbazine to dacarbazine is preferable due to its reduced side effects and improvements in fertility recovery. Crucially, these findings are of international significance and should be used to inform treatment guidelines globally to ensure patients are receiving the best treatments.”
This work is illustrative of the benefits that can be delivered through the effective translation of research into clinical practice, which will be further strengthened through the new Cambridge Cancer Research Hospital. The next steps for this research will include more long-term follow up of patients treated with eBEACOPDac, and Professor Follows hopes it will inform a global change in the guidance for treating adults with Hodgkin lymphoma.
The study was co-led by Professor George Follows, Consultant Haematologist at CUH and Dr Raheleh Rahbari, Cancer Research UK Career Development Fellow at the Wellcome Sanger Institute. The clinical research was co-ordinated by Dr Anna Santarsieri, Haematologist at CUH, supported by the Anglia Ruskin University MD Programme. The research was also supported by the Addenbrooke’s Charitable Trust (ACT), Wellcome and National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre (BRC).
Louisa’s story

Louisa, a patient in her 30’s from Peterborough, was treated with eBEACOPDac as part of the study. Three years later, she has gone on to have her second child. She said: “When I was told I had lymphoma, it was the start of the COVID pandemic and we had a new baby in the house. It was a challenging time and instinctively, what I wanted most was to get the best treatment, that would allow me to be there for my new family and do the things I love. Undergoing treatment was still difficult but I received excellent care and support throughout.”
She added: “Regaining my fertility was the most unexpected and incredible experience. I knew my chances of fertility after chemotherapy treatment would be somewhat compromised, so to have another child last year was wonderful and I am eternally grateful to be able to experience motherhood for a second time.”
Landmark ‘Pill-On-A-Thread’ cancer screening trial welcomes first participants
A pivotal clinical trial of a ‘pill-on-a-thread’ test, which will decide if it becomes a new screening programme for oesophageal cancer, has welcomed its first participant.
The BEST4 Screening trial will find out if the capsule sponge test could be used to screen people with heartburn for Barrett’s oesophagus – a condition that can lead to oesophageal cancer.
The trial is backed by £6.4 million of funding from Cancer Research UK and the National Institute for Health and Care Research (NIHR).
The capsule sponge test takes ten minutes to do and can be done by a nurse – making it much faster and less expensive than endoscopy. The trial will find out if the capsule sponge test can reduce the need for cancer treatments and prevent deaths from oesophageal cancer. The trial showcases UK science and innovation and is the last step in a series of clinical trials to see if the capsule sponge test could be offered in the cancer screening programmes of the 4 UK nations.
Over the next three years, the trial will recruit 120,000 people who regularly take medication for heartburn – the most common symptom for Barrett’s oesophagus. Barrett’s oesophagus is a precursor condition to oesophageal cancer, where cells in the food pipe start to grow abnormally.
Invitations to join the trial will be sent by text message from NHSResearch to encourage as many eligible people as possible to take part in England. Participants will be asked to join Heartburn Health, a new platform to take part in clinical trials in heartburn-linked cancers like BEST4 Screening. Mobile screening vans will be rolled out across England to deliver the tests as part of the trial.
There are around 9,300 new cases of oesophageal cancer in the UK every year, according to analysis from Cancer Research UK*. Oesophageal cancer is the seventh most common cause of cancer death in the UK, with around 22 deaths a day from the disease**.
The capsule sponge starts off as a small, coated pill attached to a thread. When a patient swallows the pill and it reaches the stomach, the coating dissolves and the sponge inside it expands to the size of a 50p coin. The sponge collects cells from the oesophagus as it is gently pulled out from the stomach by a nurse or GP. The cells are sent for testing for two proteins called Trefoil Factor 3 (TFF3), which is only found in Barrett’s oesophagus, and altered p53 protein, which identifies cells which are starting to grow out of control and become oesophageal cancer.
The trial follows decades of research by Professor Rebecca Fitzgerald and a team of scientists, clinicians and nurses at the Early Cancer Institute, University of Cambridge and Cancer Research UK Cambridge Centre, who invented and refined the capsule sponge test. The early trials were supported by the NIHR Cambridge BRC and carried out at the NIHR Cambridge Clinical Research Facility.
The future Cambridge Cancer Research Hospital will bring together clinical and research expertise, including Professor Fitzgerald’s work, under one roof. It will enable the development and discovery of more non-invasive devices like the capsule sponge, to detect cancer earlier, and save more lives.
Cancer Research UK has funded several successful clinical trials to demonstrate that the test is safe and accurate, which have been designed and run by the Cancer Research UK Cancer Prevention Trials Unit at Queen Mary, University of London. A previous clinical trial, BEST3, showed that the capsule sponge test picks up 10 times more cases of Barrett’s oesophagus in people with chronic heartburn, compared to routine GP care.
The test is faster, less invasive and far less expensive than endoscopy, which is currently used to diagnose Barrett’s oesophagus and oesophageal cancer. The test has been piloted in health services in England, Scotland and Northern Ireland for patients who are currently on waiting lists for endoscopy, because they have long-term heartburn or have been diagnosed with Barrett’s oesophagus. To date, over 24,000 capsule sponge tests have been performed in pilot programmes, helping to reduce diagnostic backlogs in endoscopy and NHS pathology.

Paul Anderson (59), a stock controller from St Neots, pictured left, is one of the first participants to join the BEST4 Screening trial in Cambridgeshire. Paul said: “I first experienced acid reflux 10 years ago and I was referred for endoscopy to get it checked out. I’ve been on medication for heartburn ever since to manage it.
“I’d never been on a clinical trial before, but when the invitation came for this one, I felt I had to sign up as the acid reflux had flared back up again. I’m hoping that it may give me some more insight into my chronic heartburn, as well as helping people who may have similar concerns about their health. I’m hopeful that playing my small part in this worthy cause will help others to get checked out earlier.”

Director of the Early Cancer Institute at the University of Cambridge, inventor of the capsule sponge test and co-principal investigator of the BEST4 trials, Professor Rebecca Fitzgerald, pictured right, said: “The capsule sponge is changing how we detect Barrett’s oesophagus and oesophageal cancer. Catching it earlier can save lives by reducing the need for chemotherapy and surgery to remove the oesophagus.
“The BEST4 Screening trial is the pinnacle of many years of painstaking research, which has demonstrated that the capsule sponge can reliably identify Barrett’s oesophagus. Thousands of people have already benefited in trials and pilot programmes, and now we’re taking the test to the next level to see if we could offer this to everyone with heartburn.
“The BEST4 Screening trial could fundamentally transform the lives of people affected by oesophageal cancer by providing the crucial evidence needed to make it a viable screening programme, rolled out to every part of the UK.”
Director of the Cancer Research UK Cancer Prevention Trials Unit at Queen Mary University of London and co-principal investigator of the BEST4 trials, Professor Peter Sasieni, said: “Most people with Barrett’s oesophagus have heartburn, but most people with heartburn don’t have Barrett’s oesophagus. We have already shown that the capsule sponge can reliably identify people with Barrett’s oesophagus. Now we need to show that using it in a targeted screening programme can help prevent oesophageal cancer and reduce deaths from this disease.
“The BEST4 Screening trial will involve over a hundred thousand people joining across the UK. It is a huge undertaking which will take many years, but it is important that we find out whether a new routine screening programme really will prevent cancers and save lives.”
Scientific Director for NIHR Programmes, Professor Danny McAuley, said: “The capsule sponge is an innovative device that has already shown great potential to prevent deaths from oesophageal cancer.
“It’s a great milestone to see the first patient recruited on this pioneering NIHR and CRUK funded trial which in future we hope can lead to routine screening for this deadly disease.
“Thousands of people are needed to join this trial, and we encourage people to sign up as participants. This important research will help benefit patients, and inform those who plan and deliver NHS services of how best to test for the disease.”
Chief Executive of Cancer Research UK, Michelle Mitchell, said: “Around 59% of all oesophageal cancer cases are preventable. Yet endoscopy, the gold standard for diagnosing and treating this cancer, is labour-intensive and not practical for a population screening programme.
“Backed by funding from Cancer Research UK, the capsule sponge has become one of the most exciting early detection tools to emerge in recent years. It’s a remarkable invention by Professor Fitzgerald and her team, and previous trials have shown how powerful it can be in identifying cancer earlier.
“Cancer Research UK is proud to be supporting this landmark clinical trial, bringing the capsule sponge test into the community and offering it to a much wider group of patients. After many decades of research, we’re on the cusp of transforming oesophageal cancer diagnosis forever.”
Minister for Public Health and Prevention, Andrew Gwynne, said: “This trial is a shining example of how we can harness the power of technology to improve patient experience and speed up diagnosis.
“This innovation has the potential to allow us to perform lifesaving screenings quicker and cheaper, freeing up vital NHS resources.
“As part of our 10 Year Health Plan to radically reform our broken NHS, we are committed to fighting cancer on all fronts, and ensuring patients have access to cutting edge, government-funded research.”
The BEST4 Screening trial is open to men over the age of 55 and women over the age of 65 who are currently taking medication for chronic heartburn. The Endosign test used in the trial is manufactured by Cyted Health, who also carry out the pathology tests on samples obtained by the capsule sponge.
More information about how to join the trial can be found at BEST4 or by contacting cuh.best4.trial@nhs.net.
You can also sign up to the NIHR’s Be Part of Research service to take part in clinical research. Simply answer a few questions about yourself and the conditions you’re interested in to be matched to studies happening in locations near you.
NIHR Cambridge theme lead elected to National Academy of Medicine
Congratulations to Professor David Rowitch, NIHR Cambridge Antenatal, Maternal and Child Health theme lead, who has been elected to the National Academy of Medicine.
Professor Rowitch’s work on glial cells (cells in the nervous system) found they provide much more than ‘the glue’ to support to the nervous system but they also protect neuron cells (nerve cells which send and receive signals from your brain).
He found their function is critical to support the spinal cord and that they also might play a part in the development of the brain and neurodegenerative illnesses.
Professor Rowitch said on being elected to the prestigious academy said: “It is a great honour to have been elected to the National Academy of Medicine.”
The National Academy of Medicine announced 90 regular members and 10 international members during its annual meeting in October. It is considered one of the highest honours in the fields of health and medicine and recognises individuals who have demonstrated outstanding professional achievement and commitment to service.
New members are elected by current members through a process that recognises individuals who have made major contributions to the advancement of the medical sciences, health care, and public health.
Victor J. Dzau, NAM President said: “This class of new members represents the most exceptional researchers and leaders in health and medicine, who have made significant breakthroughs, led the response to major public health challenges, and advanced health equity.
NMAPs Pre-application for support funding now open
Nurses, midwives and allied health professionals (NMAPs) who want to join an NIHR career development scheme are being offered the chance to apply for a pre-application support fund.
Made possible by the NIHR Cambridge BRC, this funding is open to NMAPs from the East of England which will help staff prepare for their future applications as well as support training and development needs. There will also be extra support with mentoring and any patient and public involvement projects staff may need to undertake.
Deadline for applications is on 20 November 2024 and funding for successful applicants will be from 1 December 2024 until 31 March 2025.
Applications can include the following details:
- Salary costs
- Training costs
- PPIE costs
- Mentorship costs
- Accessibility costs
More information can be found on the NIHR Cambridge BRC pre-application guidance.
To apply for funding, download a copy of the application form.
If you need further information, please contact: cuh.nmapresearch@nhs.net
MPhil in Translating Devices and Advanced Therapies Research
An exciting MPhil opportunity is available in our Devices and Advance Therapies theme to help develop your skills and knowledge. The MPhil will begin in 2025, applications close on 3 December 2024.
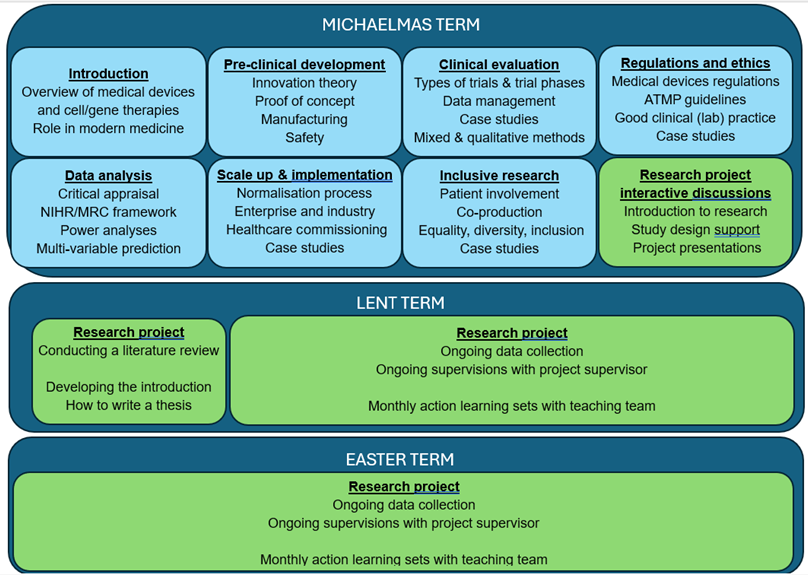
The Taught Course covers the following broad topics:
Pre-clinical development
- Innovation theory
- Proof of concept
- Manufacturing
- Safety
Clinical evaluation
- Types of trials and trial phases
- Data management
- Case studies
- Mixed and qualitative methods
Regulations and ethics
- Medical devices regulations
- AMP guidelines
- Good clinical (lab) practice
- Case studies
Data analysis
- Critical appraisal
- NIHR/MC framework
- Power analysis
- Multivariable prediction
Scale-Up and implementation
- Normalisation process
- Enterprise and industry
- Healthcare commissioning
- Case studies
Inclusive research
- Patient involvement
- Coproduction
- Equality, diversity, inclusion
- Case studies
Research project interactive discussions
- Introduction to research
- Study design support
- Project presentations
Brief overview and rationale for new course
The MPhil in ‘Translating Devices and Advanced Therapies Research’ is an exciting new course aimed at individuals who are developing or are interested in the development, obtaining regulatory approval and implementation of medical devices and/or cell and gene therapy interventions to improve patient outcomes.
This course aligns with the Cambridge Biomedical Research Centre (BRC) ‘Devices and Advanced Therapies’ theme.
Both the BRC theme and the MPhil are born out of a need expressed by researchers in clinical, academic and industry settings who do not have the skills and knowledge to develop and implement complex interventions.
The course also has an additional training option for those interested in becoming a ‘Qualified Person’ for advanced therapy interventions (the individual responsible for certifying the required standard and quality is met for cell and gene therapy for use in clinical trials).
Further information can be found on the University of Cambridge Postgraduate Study webpage or how to apply.
Contacts:
- James Tysome (Course Director)
- Matthew Smith (Course Director)
- Nick Haywood (Research Project Lead)
Cambridge researchers awarded funding to establish Advanced Therapy Hub for the East of England
Researchers at Cambridge University Hospitals (CUH) have been awarded almost £1.5 million from the National Institute for Health and Care Research (NIHR) to purchase specialist equipment required to research, test and deliver advanced therapies, such as stem cell therapies and CAR-T cell treatments for cancer, in Cambridge.
Advanced therapies are ground-breaking medicines that can involve modifying the genetic code in specific cells, replacing diseased cells with healthy ones, or using specially engineered cells to deliver targeted therapies. These treatments offer the potential for a one-time intervention to treat chronic illnesses or life-threatening conditions.
Currently, patients requiring these therapies often need to travel to the few specialist hospitals that provide them, mostly located in London. This puts them out of reach for many patients across the region whose health or other circumstances make it impossible to travel so far to receive the treatment that they need. The new funding will support the development of an Advanced Therapy Hub in Cambridge, ensuring that patients from even remote coastal areas of the East of England can benefit from these life-changing treatments, without the need for a four-hour journey to London.
The funding will be used for specialist equipment, including:
- Cell collection machinery (apheresis): Collection of stem cells (for cell and gene therapies) or immune cells (for CAR-T therapies) from a patient’s blood is an essential first step in the production of many advanced therapies. Having a research-dedicated machine will enable CUH to support an increased number of clinical trials in this area.
- Advanced therapy production equipment: Specialist equipment to produce, purify, quality-control and supply clinical-grade materials used in advanced therapies. Lack of this equipment is hampering the ability to deliver clinical trials across the UK, and the ability to produce material of the required quality in Cambridge will significantly increase our ability to deliver essential research trials for these conditions.
- Precision delivery support: Some advanced therapies require careful, targeted delivery to a specific site in the body, in order to maximise their effect while minimising side effects and complications. This funding will support imaging technology, such as ultrasound equipment and intra-operative MRI that can guide clinicians in delivering these new therapies with pinpoint accuracy.
In collaboration with the NIHR, the University of Cambridge, and industry partners, the hub will advance research and development of new therapies, including for rare diseases, auto-immune diseases, cancer and neurodegenerative conditions.
The Advanced Therapy Hub will bring transformative benefits to the region by increasing local capacity for clinical trials in regenerative medicine and immunotherapy. This will expand access to these cutting-edge treatments across the East of England.
Dr Ben Uttenthal, consultant haematologist at CUH commented: “We’re delighted to have secured this funding. Over the last few years in Cambridge we have successfully treated many patients from across the East of England with cell and gene therapies that have been made elsewhere. This equipment will take us to the next level, allowing us to make our own completely new advanced therapies in partnership with the University and then test them in world-first clinical trials.”
Professor Miles Parkes, director of the NIHR Cambridge Biomedical Research Centre, commented: “This support from the NIHR will make a critical difference to our ability to design and deliver truly innovative trials of new advanced therapies. Given the historic strengths in Cambridge relating to genetics and discovery science there is a wealth of opportunity to bring this to clinical impact through this support.”
Sarah Albon, director of the Cambridge Cellular Therapy Laboratory (CCTL) said: “Currently we have a wealth of scientific and clinical expertise on the Cambridge Biomedical Campus. This grant will aid the missing piece of the puzzle and enhance our ability to translate novel research from bench to bedside and manufacture life-saving therapies here on the patient’s doorstep.”
Professor John Bradley, director of research at CUH added: “These state-of-the-art facilities will support innovative research that will be undertaken in partnership with organisations across the region, strengthening the East of England’s research ecosystem.”
Cambridge and GSK announce new five-year collaboration to help patients with hard-to-treat kidney and respiratory diseases
GSK plc is making a five-year investment to establish the Cambridge-GSK Translational Immunology Collaboration (CG-TIC), with the University of Cambridge and Cambridge University Hospitals. The collaboration is focused on understanding the onset of a disease, its progression, how patients respond to therapies and on developing biomarkers for rapid diagnosis. Ultimately, the goal is to trial more effective, personalised medicines.
The collaboration will focus on kidney and respiratory diseases, both of which affect large numbers of people worldwide. Kidney disease is estimated to affect 850 million people (roughly 10% of the world’s population) (International Society of Nephrology) and chronic respiratory diseases around 545 million (The Lancet).
Many types of kidney disease remain poorly understood and treatments, where they exist, tend to have limited efficacy. Chronic kidney disease is particularly unpleasant and debilitating for patients, often leading to end-stage disease. Treatments such as transplant and dialysis involve complex medical regimes and frequent hospital visits, making effective prevention and treatment the aim.
To make progress in treating these challenging disease areas, CG-TIC will apply an array of new techniques, including the use of cutting-edge single cell technologies to characterise how genes are expressed in individual cells. AI and machine learning have a critical role to play in transforming how data is combined and interrogated.
Using these techniques, the ambition is to be able to initiate new studies and early phase trials of new therapies for a number of hard-to-treat diseases which affect the kidneys. The same techniques will be applied to respiratory diseases and findings will be shared across the disease areas to potentially help identify and share better treatments across these different targets.
Tony Wood, Chief Scientific Officer, GSK, said: “Collaboration is at the heart of scientific progress and is fundamental to how we do R&D at GSK. We’re excited to build on our existing work with the University of Cambridge to further this world-leading scientific and technological capability in the UK. By bringing together Cambridge’s expertise and our own internal capabilities, including understanding of the immune system and the use of AI to accelerate drug development, we have an opportunity to help patients struggling with complex disease.”
The aim of CG-TIC is to improve outcomes for patients and Cambridge provides a unique environment in which to involve them, with Cambridge University Hospitals playing a pivotal role in the collaboration and Royal Papworth Hospital NHS Foundation Trust, the UK’s leading heart and lung hospital, a likely future partner.
Home to the hospitals and to much of the collaboration’s research activity, the Cambridge Biomedical Campus provides a unique environment where academia, industry and healthcare can come together and where human translational research is supported by the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre.
Professor Deborah Prentice, Vice-Chancellor of the University of Cambridge, added: “The University sits at the heart of Europe’s leading life sciences cluster, where excellent research and the NHS’s clinical resources combine with the talent generated by the many innovative bioscience companies that call Cambridge home. Through this very important collaboration with GSK, Cambridge will be able to drive economic growth for the UK while improving the health of people in this country and around the world.”
Roland Sinker, CEO of Cambridge University Hospitals NHS Foundation Trust, welcomed the collaboration, adding: “We are very excited to be part of this important partnership, which is another example of Cambridge experts working together to develop transformational new therapies, and use existing ones more precisely, to improve outcomes for patients with chronic and debilitating conditions.”
The Cambridge-GSK Translational Immunology Collaboration will be co-led by Nicolas Wisniacki, VP, Clinical Research Head, GSK, and David Thomas, Professor of Renal Medicine, University of Cambridge and principal investigator at the Cambridge Institute for Therapeutic Immunology and Infectious Diseases
New evidence about whether Air Cleaning Units reduce hospital-acquired infections
Cambridge researchers have been looking at whether Air Cleaning Units in hospital wards reduce hospital-acquired infections, particularly COVID-19.

Previous research conducted at Cambridge University Hospitals had demonstrated Air Cleaning Units can remove the virus causing COVID-19 from the air, but it was unknown whether this would lead to a reduction in hospital-acquired infections.
In a newly published study in the Journal of Hospital Infection, supported by the NIHR Cambridge BRC, found the number of hospital-acquired infections were recorded on four wards in the Department of Medicine for the Elderly at Addenbrooke’s Hospital in Cambridge.
In two of the wards, study Air Cleaning Units were installed, while the other two wards continued with usual practice. The researchers compared the number of hospital-acquired infections in both pairs of wards to find out whether or not fewer hospital-acquired infections occurred when Air Cleaning Units were present.
The researchers from the Department of Medicine at Cambridge University Hospitals and and the MRC Biostatistics Unit (BSU), found that having Air Cleaning Units in place was associated with a 22% lower risk of hospital-acquired COVID-19 virus infection, during a hospital stay of typical duration. This suggests Air Cleaning Units may reduce infections to a clinically-meaningfully degree. However, a larger study would be needed to confirm results as there was large uncertainty in this finding: the current results do not rule out Air Cleaning Units having no impact or even increasing the risk.
Researchers found the Air Cleaning Units were initially well received. However, Units were increasingly not operational during the second intervention ward near the end of the study, in Summer 2022. This may have contributed to study results being less certain than expected and most likely occurred because the Units were switched off, perhaps due to noise. The study’s survey of staff and patients found that noise from the Air Cleaning Units was moderately disturbing on the second intervention ward. The survey also found that the majority of survey respondents did not associate the Air Cleaning Units with infection prevention and control and the survey helped us understand how we might be able to better implement this technology in the future.
Dr Victoria Keevil, Consultant Geriatrician at Cambridge University Hospitals and Senior Research Associate in the Clinical School’s Department of Medicine, who led the study and said: “Although challenging, it was important to evaluate this technology in a real-world setting and include the experiences of staff and patients. Many new treatments and technologies do not achieve their potential to improve patient care, even if efficacy is proven, because not enough research considers whether they can be easily adopted into everyday clinical practice.
It is also important to highlight that this study was supported by a research team with diverse skills. Working together was key to making the best use of the available resources enabling this research.”
Research paper:
Brock et al. Efficacy of Air Cleaning Units for preventing SARS-CoV-2 and other hospital-acquired infections on medicine for older people wards: A quasi-experimental controlled before-and- after study. Journal of Hospital Infection. https://doi.org/10.1016/j.jhin.2024.09.017
Ultra-powered MRI scans show damage to brain’s ‘control centre’ is behind long-lasting Covid-19 symptoms
Damage to the brainstem – the brain’s ‘control centre’ – is behind long-lasting physical and psychiatric effects of severe Covid-19 infection, a study suggests.
Using ultra-high-resolution scanners that can see the living brain in fine detail, researchers from the Universities of Cambridge and Oxford and supported by NIHR Cambridge and Oxford BRCs, were able to observe the damaging effects Covid-19 can have on the brain.
The study team scanned the brains of 30 people who had been admitted to hospital with severe Covid-19 early in the pandemic, before vaccines were available. The researchers found that Covid-19 infection damages the region of the brainstem associated with breathlessness, fatigue and anxiety.
The powerful MRI scanners used for the study, known as 7-Tesla or 7T scanners, can measure inflammation in the brain. Their results, published in the journal Brain, will help scientists and clinicians understand the long-term effects of Covid-19 on the brain and the rest of the body. Although the study was started before the long-term effects of Covid were recognised, it will help to better understand this condition.
The brainstem, which connects the brain to the spinal cord, is the control centre for many basic life functions and reflexes. Clusters of nerve cells in the brainstem, known as nuclei, are responsible for regulating and processing essential bodily functions such as breathing, heart rate, pain and blood pressure.
“Things happening in and around the brainstem are vital for quality of life, but it had been impossible to scan the inflammation of the brainstem nuclei in living people, because of their tiny size and difficult position.” said first author Dr Catarina Rua, from the Department of Clinical Neurosciences. “Usually, scientists only get a good look at the brainstem during post-mortem examinations.”
“The brainstem is the critical junction box between our conscious selves and what is happening in our bodies,” said Professor James Rowe, also from the Department of Clinical Neurosciences, who co-led the research. “The ability to see and understand how the brainstem changes in response to Covid-19 will help explain and treat the long term effects more effectively.”
In the early days of the Covid-19 pandemic, before effective vaccines were available, post-mortem studies of patients who had died from severe Covid-19 infections showed changes in their brainstems, including inflammation. Many of these changes were thought to result from a post-infection immune response, rather than direct virus invasion of the brain.
“People who were very sick early in the pandemic showed long-lasting brain changes, likely caused by an immune response to the virus. But measuring that immune response is difficult in living people,” said Rowe. “Normal hospital type MRI scanners can’t see inside the brain with the kind of chemical and physical detail we need.”
“But with 7T scanners, we can now measure these details. The active immune cells interfere with the ultra-high magnetic field, so that we’re able to detect how they are behaving,” said Rua. “Cambridge was special because we were able to scan even the sickest and infectious patients, early in the pandemic.”
Many of the patients admitted to hospital early in the pandemic reported fatigue, breathlessness and chest pain as troubling long-lasting symptoms. The researchers hypothesised these symptoms were in part the result of damage to key brainstem nuclei, damage which persists long after Covid-19 infection has passed.
The researchers saw that multiple regions of the brainstem, in particular the medulla oblongata, pons and midbrain, showed abnormalities consistent with a neuroinflammatory response. The abnormalities appeared several weeks after hospital admission, and in regions of the brain responsible for controlling breathing.
“The fact that we see abnormalities in the parts of the brain associated with breathing strongly suggests that long-lasting symptoms are an effect of inflammation in the brainstem following Covid-19 infection,” said Rua. “These effects are over and above the effects of age and gender, and are more pronounced in those who had had severe Covid-19.”
In addition to the physical effects of Covid-19, the 7T scanners provided evidence of some of the psychiatric effects of the disease. The brainstem monitors breathlessness, as well as fatigue and anxiety. “Mental health is intimately connected to brain health, and patients with the most marked immune response also showed higher levels of depression and anxiety,” said Rowe. “Changes in the brainstem caused by Covid-19 infection could also lead to poor mental health outcomes, because of the tight connection between physical and mental health.”
The researchers say the results could aid in the understanding of other conditions associated with inflammation of the brainstem, like MS and dementia. The 7T scanners could also be used to monitor the effectiveness of different treatments for brain diseases.
“This was an incredible collaboration, right at the peak of the pandemic, when testing was very difficult, and I was amazed how well the 7T scanners worked,” said Rua. “I was really impressed with how, in the heat of the moment, the collaboration between lots of different researchers came together so effectively.”
Professor Miles Parkes, director of the NIHR Cambridge BRC said: “In identifying subtle changes in the brainstem using the latest MRI scanning technology this important research helps shed light on the difficult to characterise but very real longer term consequences of COVID infection. Patients who experience these symptoms will be relieved to see progress being made in understanding them. The work was supported by the NIHR Biomedical Research Centres in Cambridge and Oxford, and shows the power of collaboration between these two centres of excellence to deliver important scientific breakthroughs”
The research was supported in part by the NIHR Cambridge Biomedical Research Centre, the NIHR Oxford Biomedical Research Centre, and the University of Oxford COVID Medical Sciences Division Rapid Response Fund.
Reference:
Catarina Rua et al. ‘7-Tesla quantitative susceptibility mapping in COVID-19: brainstem effects and outcome associations.’ Brain (2024). DOI: 10.1093/brain/awae215
Free public event on our latest inflammation research
WHERE: Hexagon Room, Frank Lee Centre, Cambridge Biomedical Campus
Inflammation is an essential part of how we fight off infection. However, sometimes it persists even after an infection clears, happens in the wrong place or kicks off even without any signs of infection – and results in a range of health conditions that reduce quality of life for patients.
Join us for an hour of talks either in the afternoon (4-5pm) or evening (6-7pm) on the latest developments in inflammation research at NIHR Cambridge BRC and take the opportunity to chat with local researchers about their research.
Whether you choose to come for the afternoon or evening talks, you are welcome to spend the hour from 5-6pm chatting to our researchers about their work and view the research posters on display. Why not vote for your favourite poster while enjoying some light refreshments?
Our speakers
- Dr. Bina Patel, Clinical Research Fellow, Department of Clinical Neuroscience at the University of Cambridge
- Prof. Menna Clatworthy, NIHR Cambridge BRC Theme Lead for Immunity, Inflammation and Transplantation and Professor of Translational Immunology, University of Cambridge
- Prof. Clare Elizabeth Bryant, Professor of Innate Immunity, Department of Medicine at the University of Cambridge
Book your preferred session
You only need to book one session to attend the talks and poster exhibition, as the talks are the same for the afternoon and evening slots: and you are welcome to stay for some or all of the hour (5-6pm) between talks to chat to researchers, view the posters and enjoy light refreshments.
- To book the afternoon session from 4-5pm: visit https://bit.ly/InflamAFTER
- To book the evening session from 6-7pm: visit https://bit.ly/InflamEVE
Questions?
If you have any questions either before or after you book your ticket, please contact Georgina Norris via email: gan23@cam.ac.uk.
Global spotlight on Cambridge heart trial
Results of an NIHR Cambridge BRC and NIHR Cambridge CRF supported trial which may help prevent patients from having repeat heart attacks, will be presented at the 30th European Society of Cardiology (ESC) Congress, the largest cardiology conference globally.
The IVORY and IVORY FINALE trial looked at whether a low dose of the cancer drug, known as aldesleukin, could increase the activation of immune cells shown to protect the heart and help patients who have had a heart attack.
Current treatment for heart attacks centres on the re-establishment and maintenance of blood flow in the coronary arteries using blood thinners, with or without stents, as well as cholesterol lowering medication. Despite current optimal therapy, heart attacks can re-occur.
The team believes the immune system is an important process in the development of atherosclerosis (plaque disease in arteries) which has not been directly harnessed in these patients and attempted to target it using a novel approach.
The researchers found in a previous trial that low doses of aldesleukin, a drug normally used to treat kidney cancer (at much higher doses), stimulates the production of protective immune cells, called Tregs safely in patients with heart attacks.
In the current trial, they tested to see if the drug reduced inflammation in the arteries of patients after an initial heart attack, as inflammation in the arteries has previously been associated with an increase in the risk of recurrent heart attacks and death.
The study was led by researchers at Cambridge University Hospitals (CUH) and the University of Cambridge (UofC) as well as Royal Papworth Hospital, and was supported by the Medical Research Council, the British Heart Foundation, the NIHR Cambridge Biomedical Research Centre and the NIHR Cambridge Clinical Research Facility.

The results will be unveiled at the ‘late-breaking’ sessions of the 30th European Society of Cardiology (ESC) Congress 2024 in London on 30 August and will be presented by Dr Rouchelle Sriranjan, an interventional cardiology registrar and NIHR clinical lecturer in cardiology, pictured right.
CUH consultant clinical pharmacologist, affiliated associate professor and trial chief investigator, Dr Joseph Cheriyan, said: “We are delighted to be presenting these novel data at the ESC and particularly in the late breaking session, which is a highlight of the conference.
“This study was the product of very intense work by many different teams on the campus in very sick patients and demonstrates the importance of collaborative teamwork. We are very grateful to the patients for their time and dedication to our trial.”
Dr Stephen Hoole was the trial lead for Royal Papworth Hospital, whilst Professor Rudd led the imaging component for the trial.
Professor Ziad Mallat, BHF professor of cardiovascular medicine, UofC, who conceived the trial said: “If positive, this is potentially a new treatment approach that boosts our immune defence mechanisms to tame heart attacks. It could also be an important step forward in the treatment of patients with heart attacks which needs continued exploration.
“Our results should be of interest to the wider cardiovascular community. In addition to our supporters and funders, we would like to thank everyone who has worked so hard on the trial, especially the patients who have taken part.”
Cambridge professor receives top award for outstanding research in endocrinology
Congratulations to Krishna Chatterjee, Director of the NIHR Cambridge Clinical Research Facility and Professor of Endocrinology at the University of Cambridge, who has been awarded the Gerald D. Aurbach Award for Outstanding Translational Research from the Endocrine Society.
Prof Chatterjee is one of 14 leading endocrinologists who were selected for the Society’s prestigious 2025 Laureate Awards, which recognise the highest achievements in endocrinology research and care, and which are regarded as the highest honours in the field.
Prof Chatterjee was awarded for his research on genetic and molecular endocrinology, exploring disorders including resistance to thyroid hormone and PPARgamma gene defects associated with lipodystrophic insulin resistance.
He said: “It is a privilege to accept this award which also represents the efforts of many clinical and scientist colleagues as well as patients taking part in our research, without whom our contributions to advancing knowledge and changing health outcomes in endocrine disorders would not have been possible.”
The Endocrine Society will present the awards to the winners at ENDO 2025, the Society’s annual meeting, being held next July 12-15 in San Francisco.
The role of a SAB and why it’s important
A personal reflection from Professor Miles Parkes, Director of NIHR Cambridge Biomedical Research Centre (BRC)
Every two years something very special happens in the life of the NIHR Cambridge BRC – and it’s something we all look forward to hugely.
It’s when our international Scientific Advisory Board (or SAB) of leaders in biomedical research join us over two days as ‘critical friends’, to hear about our early translational research (research fresh from the laboratory, exploring how it can be applied in people to sustain health and treat disease), review its quality and provide strategic direction. The meeting also provides an opportunity for us and our partners to come together to celebrate our successes and take a critical look at ourselves. This whole process is crucial, not only for our governance, but to hold us accountable for the research we do.
In April this year, meeting in the Cancer Research UK Cambridge Institute, I welcomed our SAB and a large audience with a brief introduction to the biomedical campus with its rich inter-digitation of healthcare, research, and industry facilities. The BRC plays a key role in supporting early translational research infrastructure, enabling projects led by our investigators and their partners. Many of these projects involve extensive collaborations and reach beyond the campus to our region and nationally.
After my introduction I handed over to the leads of our 13 research themes for a sequence of presentations on their ground-breaking research and its impact. Their emphasis is absolutely on priorities identified by patients, and how our research can best address their concerns and health priorities. None of our research would happen without patients – and so we had talks on our patient and public involvement and equality and diversity work, and an opportunity for the SAB and others to see first-hand the new Clinical Research Facility (a place for participants to take part in research) in the Victor Phillip Dahdaleh Heart and Lung Research Institute.
Over two days, we enjoyed lots of opportunities to ask questions and give feedback – including where we can improve. There was lots of thought-provoking discussion about the direction of research and how we can develop new diagnostics and treatments for some of our biggest health challenges.
Having an event like this is critical to our work – highlighting the successes but also providing an opportunity for constructive criticism. I was delighted to see the SAB members so engaged, see so many staff in the audience – and receive so many positive comments regarding the important research we do in Cambridge.
But please don’t just take my word for it: watch our short video and hear for yourself why our attendees think that our biomedical campus supported by the NIHR Cambridge BRC is a special place to conduct health research.
Patient and Public Involvement week returns and is a huge hit
Nearly 200 people attended an exciting week of Patient and Public Involvement events at the end of June. This was the first event of its kind held on the Cambridge Biomedical Campus.
Hosted by the NIHR Cambridge Biomedical Research Centre (BRC), University of Cambridge and Anglian Ruskin University (ARU), the event aimed to celebrate and raise awareness of Patient and Public Involvement (PPI) in health research.
The week began with a webinar for members of the public who were able to hear how people’s voices can help shape health and care research and opportunities to get involved in research through CUH, NIHR Cambridge BRC, Anglia Ruskin University and other health and research partners across the region. The 90-minute webinar highlighted the importance of PPI in health research and attendees heard about some of the examples of activities the NIHR Cambridge BRC PPI panel members are involved in.
On Tuesday we held a public PPI showcase of talks and poster exhibits from health researchers where they explained how patient and public involvement had made an impact to their research. Attendees were able to network and vote for the best poster and talks from the morning and afternoon sessions. The all-day event received excellent feedback, with one saying: “I just wanted to give a big thank you. It’s really important to have an event where you can find out about what is going on in Cambridge and the PPI space.”
Wednesday provided an opportunity to learn from our partners at ARU about their ‘Let’s Shape Research Together’ public involvement programme. Their annual conference showcases case studies, as well as keynote speakers from PPIE experts across Cambridgeshire.
The final event of the week was a market stall drop-in at the David Dunn Suite in CUH, where staff and members of the public could meet health and research patient and public involvement teams across Cambridgeshire, from the NIHR Cambridge BRC, Cambridge Biomedical Campus local voices, CRUK, IMS and Stem Cell, Cambridge School of Biology, Cambridge Clinical School, Cambridge Children’s Hospital, ACTIVE and CUH members. The organisations were able to provide more information on how people could volunteer to their panels and offer staff support and advice on their PPI projects. One attendee said: “It was a great event, I had some really valuable conversations with the staff.”
Dr Amanda Stranks, PPI and communications strategy lead from NIHR Cambridge BRC and one of the organisers said: “This week of PPI events gave us the chance to celebrate and showcase some of the best examples of patient and public involvement in research that are happening on the Cambridge Biomedical Campus. The variety and quality of the examples are testament to how researchers and our public are working in partnership across Cambridge to produce life-changing research.
“Patient and Public Involvement makes such a difference to the research we do here in Cambridge. We need public voices to make sure we are doing the best research we can, and that our research opportunities are widely accessible and meet patient’s needs. Showcasing examples of how the public make a different to our research and our researchers helps to encourage more researchers and members of the public to get involved.
“We’re really pleased by the response of staff and the public to the week’s activities and would like to extend our thanks to all who came and supported the events. We received some amazing feedback and are delighted to see an increase in sign-ups to some of the Cambridgeshire panels. We’re looking forward to seeing everyone next year, for an even bigger regional public involvement week!
Read about the three winners from the PPI showcase as voted by attendees.
Winners announced for 2024’s Cambridge PPI showcase event
Improving women’s access to pelvic health services, using drama and theatre to help interpret genetic data research and public engagement with paediatric Inflammatory Bowel Disease were all winners at the 2024 Cambridge Patient and Public Involvement (PPI) showcase.
Jointly hosted by the NIHR Cambridge BRC (BRC) and the University of Cambridge, more than 115 members of the public, researchers and healthcare staff attended a showcase of how patient and public involvement (PPI) has impacted our Cambridge research.
The day was split into three sessions of morning and afternoon talks and a poster exhibition over lunch, with a chance to network with researchers. Attendees were then able to vote for their favourite poster and talk from each session, with the winners receiving a certificate and £50 gift voucher.
Improving disadvantaged women’s access to pelvic healthcare

The winning talk from the morning session was awarded to Claire Brown, pre-doctoral research fellow and pelvic health physiotherapist at Cambridge University Hospitals, pictured left, and Dr Caroline Zwierzchowska-Dod, Strategic Lead, Cambridgeshire and Peterborough Maternity and Neonatal Voices. Their research focused on whether women in under-served communities were able to access pelvic health services and how much information they had received about pelvic floor dysfunction during their pregnancy.
Claire and Caroline worked with leaders from a range of community groups on how best to approach women to take part. This led to a series of listening events with an ethnic minority mothers’ group, a Gypsy-Roma-Traveller (GRT) community and with asylum seekers living in Government accommodation. In total 18 women attended to share their ideas, questions and experiences.
Claire and Caroline found most women had not received enough information on the risks of developing pelvic floor dysfunction during pregnancy and were therefore less likely to recognise their symptoms in order to find support or treatment. The women preferred to speak to their GP or midwife, but these clinical teams may not have specific expertise to help manage the condition effectively. Most of the women that Claire and Caroline spoke to also said they were unaware that there were self-referral options for pelvic health physiotherapy.

From these sessions Claire and Caroline were able to create a leaflet, including an easy read format, of pelvic floor muscle exercises and post-caesarean advice for healthcare staff in hospitals and the community to give to their patients and make them more aware of the disorder.
Claire said: “I have been very lucky to be able to work with Caroline, thanks to a PPIE innovation grant from the NIHR CRN East of England, which covered the costs of these listening events. We have discovered invaluable information and acted on this to improve the access to pelvic health services, this is just the beginning of this journey. I am so pleased we have been able to build strong relationships with community leaders for future work.”
Playing with Genetic data

The winning talk from the afternoon session was awarded to Dr Amy Mason, research associate in cardiovascular disease, pictured left. Amy’s talk explained the challenges in talking with the public about her research using genetic data to predict whether someone was at a potential risk of cardiovascular disease (conditions that affect the heart) in the future.
Amy worked with Cambridge Creative Encounters and local theatre group Babolin Young Company to develop two short fictional plays about whether knowing about being at risk of a condition could change a patient’s lifestyle and environmental behaviours.
The PPI group, made up of diverse representatives, reviewed the plays and information sheets to create a discussion about genetic risk scores. The plays helped the panel to explore the topic much more personally and reflectively, rather than just looking at facts, data and statistics. The drama helped the participants to come up with questions, such as whether the data was accurate, does it help patients and is that knowledge worthwhile? They also discussed how precise genetic risk scores are and how the data sets are collected. The health data could be different for each diverse group and it wouldn’t necessarily mean a ‘one size fits all’.
Amy found that using more creative outputs such as plays helped improve conversations and was an effective way of getting key messages across.
Amy said: “I really loved working with our PPIE volunteers with creative arts. I found talking about fictional narratives with the panel made me more confident to bring up my own concerns and questions. The panel’s responses really changed how I prioritise and approach research questions.”


You can watch the plays at 57:40 minutes in , there is also a short documentary about creative encounters behind the curtains.
Driving inclusivity with IBD

There were 12 posters on display during the lunchtime exhibition and attendees voted for research nurse, Claire Glemas, pictured right, from the Cambridge Stem Cell Institute as the poster winner.
Claire’s poster highlighted her PPI work with children with Inflammatory Bowel Disease (IBD), particularly focusing on understanding their lived experiences. By working with a group of children and young people, she wanted to see where improvements could be made with the patient’s experience during their clinic appointments and when taking part in research.
Over three years, Claire and a PPI group of children and young people developed a number of engagement opportunities including a quarterly gastroenterology newsletter, co-designed and edited with a member of the group. They hosted a Young Scientist day where children can come and see the labs and work as research assistants, and an annual family day with research, hospital staff and volunteers. Patients were given access to IBDmate, an educational tool about IBD, and an interactive colouring journal to help with their treatment pathway.
In her poster, Claire demonstrates the importance of communicating effectively with patients and their parents. She hopes that her engagement work with the children’s group will help new patients and their families understand IBD and make informed decisions about their health and when taking part in research. It has created an engaged community of staff and patients working together to improve healthcare services for young patients with IBD.
Claire said: “Our patient community will ensure every aspect of our research is shaped by the perspective and voices of our patients and families. We are committed to our mission, building PPI into an integral part of what we do, gathering invaluable insights and ultimately driving improvement in our research.”
The PPI Showcase was the first on the Cambridge Biomedical Campus, with more than 25 talks and posters highlighting how PPI makes a difference in local research. Dr Amanda Stranks, PPI and communications strategy lead for the NIHR Cambridge BRC who led the event said: “The PPI showcase was a wonderful opportunity to show researchers and the public how PPI has shaped our research and made a positive impact in the way we deliver our studies. There was such a variety of examples on display, demonstrating the importance of PPI and how it makes a difference. We’re looking forward to making this an annual event, to encourage more people to get involved in our research!’
Georgina Norris, PPI coordinator for the NIHR Cambridge BRC and event organiser said: “We were overwhelmed with the number of abstracts we received for people who wanted to take part, so we know there truly is some amazing PPI work happening across Cambridge.
“All the talks and posters on the day were brilliant but sadly we could only award one person from each session. I’d like to thank everyone who presented their PPI work, all our attendees and everyone who has contributed to our research, we couldn’t do it without you.”
Artificial intelligence outperforms clinical tests at predicting progress of Alzheimer’s disease
Cambridge scientists have developed an artificially-intelligent tool capable of predicting in four cases out of five whether people with early signs of dementia will remain stable or develop Alzheimer’s disease.
The team say this new approach could reduce the need for invasive and costly diagnostic tests while improving treatment outcomes early when interventions such as lifestyle changes or new medicines may have a chance to work best.
Dementia poses a significant global healthcare challenge, affecting over 55 million people worldwide at an estimated annual cost of $820 billion. The number of cases is expected to almost treble over the next 50 years.
The main cause of dementia is Alzheimer’s disease, which accounts for 60-80% of cases. Early detection is crucial as this is when treatments are likely to be most effective, yet early dementia diagnosis and prognosis may not be accurate without the use of invasive or expensive tests such as positron emission tomography (PET) scans or lumbar puncture, which are not available in all memory clinics. As a result, up to a third of patients may be misdiagnosed and others diagnosed too late for treatment to be effective.
A team led by scientists from the Department of Psychology at the University of Cambridge has developed a machine learning model able to predict whether and how fast an individual with mild memory and thinking problems will progress to developing Alzheimer’s disease. In research published today in eClinical Medicine, they show that it is more accurate than current clinical diagnostic tools.
To build their model, the researchers used routinely-collected, non-invasive, and low-cost patient data – cognitive tests and structural MRI scans showing grey matter atrophy – from over 400 individuals who were part of a research cohort in the USA.
They then tested the model using real-world patient data from a further 600 participants from the US cohort and – importantly – longitudinal data from 900 people from memory clinics in the UK and Singapore.
The algorithm was able to distinguish between people with stable mild cognitive impairment and those who progressed to Alzheimer’s disease within a three-year period. It was able to correctly identify individuals who went on to develop Alzheimer’s in 82% of cases and correctly identify those who didn’t in 81% of cases from cognitive tests and an MRI scan alone.
The algorithm was around three times more accurate at predicting the progression to Alzheimer’s than the current standard of care; that is, standard clinical markers (such as grey matter atrophy or cognitive scores) or clinical diagnosis. This shows that the model could significantly reduce misdiagnosis.
The model also allowed the researchers to stratify people with Alzheimer’s disease using data from each person’s first visit at the memory clinic into three groups: those whose symptoms would remain stable (around 50% of participants), those who would progress to Alzheimer’s slowly (around 35%) and those who would progress more rapidly (the remaining 15%). These predictions were validated when looking at follow-up data over 6 years. This is important as it could help identify those people at an early enough stage that they may benefit from new treatments, while also identifying those people who need close monitoring as their condition is likely to deteriorate rapidly.
Importantly, those 50% of people who have symptoms such as memory loss but remain stable, would be better directed to a different clinical pathway as their symptoms may be due to other causes rather than dementia, such as anxiety or depression.
Senior author Professor Zoe Kourtzi from the Department of Psychology at the University of Cambridge said: “We’ve created a tool which, despite using only data from cognitive tests and MRI scans, is much more sensitive than current approaches at predicting whether someone will progress from mild symptoms to Alzheimer’s – and if so, whether this progress will be fast or slow.
“This has the potential to significantly improve patient wellbeing, showing us which people need closest care, while removing the anxiety for those patients we predict will remain stable. At a time of intense pressure on healthcare resources, this will also help remove the need for unnecessary invasive and costly diagnostic tests.”
While the researchers tested the algorithm on data from a research cohort, it was validated using independent data that included almost 900 individuals who attended memory clinics in the UK and Singapore. In the UK, patients were recruited through the Quantiative MRI in NHS Memory Clinics Study (QMIN-MC) led by study co-author Dr Timothy Rittman at Cambridge University Hospitals NHS Trust and Cambridgeshire and Peterborough NHS Foundation Trusts (CPFT).
The researchers say this shows it should be applicable in a real-world patient, clinical setting.
Dr Ben Underwood, Honorary Consultant Psychiatrist at CPFT, assistant professor at the Department of Psychiatry, University of Cambridge and NIHR Cambridge BRC researcher, said: “Memory problems are common as we get older. In clinic I see how uncertainty about whether these might be the first signs of dementia can cause a lot of worry for people and their families, as well as being frustrating for doctors who would much prefer to give definitive answers. The fact that we might be able to reduce this uncertainty with information we already have is exciting and is likely to become even more important as new treatments emerge.”
Professor Kourtzi said: “AI models are only as good as the data they are trained on. To make sure ours has the potential to be adopted in a healthcare setting, we trained and tested it on routinely-collected data not just from research cohorts, but from patients in actual memory clinics. This shows it will be generalisable to a real-world setting.”
The team now hope to extend their model to other forms of dementia, such as vascular dementia and frontotemporal dementia, and using different types of data, such as markers from blood tests.
Professor Kourtzi added: “If we’re going to tackle the growing health challenge presented by dementia, we will need better tools for identifying and intervening at the earliest possible stage. Our vision is to scale up our AI tool to help clinicians assign the right person at the right time to the right diagnostic and treatment pathway. Our tool can help match the right patients to clinical trials, accelerating new drug discovery for disease modifying treatments.”
The study was funded by Wellcome, the Royal Society, Alzheimer’s Research UK, the Alzheimer’s Drug Discovery Foundation Diagnostics Accelerator, the Alan Turing Institute, and the National Institute for Health and Care Research Cambridge Biomedical Research Centre.
Paper:
Lee, LY & Vaghari, D et al. Robust and interpretable AI-guided marker for early dementia prediction in real-world clinical settings. eClinMed; 12 July 2024; DOI: 10.1016/j.eclinm.2024.102725
Researcher awarded prestigious Academy of Medical Sciences Fellowship
Congratulations to Professor Nita Forouhi, NIHR Senior Investigator and co-theme lead for Nutrition, Obesity, Metabolism and Endocrinology, who has been elected as a fellow of the Academy of Medical Sciences.
Professor Forouhi’s work focuses on nutritional epidemiology and finding modifiable factors for the prevention of type 2 diabetes and related metabolic disorders.
The Academy of Medical Sciences is the independent, expert body representing the diversity of medical science in the UK. Its mission is to advance biomedical and health research and its translation into benefits for society. The Academy’s elected Fellows are the most influential scientists in the UK and worldwide, drawn from the NHS, academia, industry and the public service.
In total the academy has recognised 58 scientists for this year’s award. They were selected for their outstanding contribution and discoveries to biomedical and health sciences.
Professor Andrew Morris PMedSci, President of the Academy of Medical Sciences, said: “It is an honour to welcome these brilliant minds to our Fellowship. Our new Fellows lead pioneering work in biomedical research and are driving remarkable improvements in healthcare. We look forward to working with them, and learning from them, in our quest to foster an open and progressive research environment that improves the health of people everywhere through excellence in medical science.
“It is also welcoming to note that this year’s cohort is our most diverse yet, in terms of gender, ethnicity and geography. While this progress is encouraging, we recognise that there is still much work to be done to truly diversify our Fellowship. We remain committed to our EDI goals and will continue to take meaningful steps to ensure our Fellowship reflects the rich diversity of the society we serve.”
Read the full list of this years of elected scientists.
The new Fellows will be formally admitted to the Academy on Wednesday 18 September 2024.
Cambridge researchers elected as Fellows of the Royal Society 2024
Congratulations to our NIHR Cambridge BRC researchers who have been elected as Fellows of the Royal Society, the UK’s national academy of sciences.
More than 90 researchers from around the world have been elected as Fellows of the Royal Society and have been recognised for their invaluable contributions to science.
This year’s list included four NIHR Cambridge BRC researchers, (pictured left to right):
Professor Sarah-Jayne Blakemore, Professor of Psychology and Cognitive Neuroscience, from the NIHR Cambridge BRC Mental Health theme.
Professor Rebecca Fitzgerald, Professor of Cancer Prevention and Director, Early Cancer Institute and Devices and Advanced Therapies NIHR Cambridge BRC theme lead.
Professor Patrick Chinnery, Professor of Neurology, Department of Clinical Neurosciences, from the NIHR Cambridge BRC Neurosciences theme.
Professor George Malliaras, Professor of Technology, Department of Engineering, University of Cambridge, from the NIHR Cambridge BRC Devices and Advanced Therapies theme.
Sir Adrian Smith, President of the Royal Society, said: “I am pleased to welcome such an outstanding group into the Fellowship of the Royal Society.
“This new cohort have already made significant contributions to our understanding of the world around us and continue to push the boundaries of possibility in academic research and industry.
“From visualising the sharp rise in global temperatures since the industrial revolution to leading the response to the Covid-19 pandemic, their diverse range of expertise is furthering human understanding and helping to address some of our greatest challenges.
“It is an honour to have them join the Fellowship.”
Read the full list from the Royal Society.
Baby born deaf can hear after breakthrough gene therapy
A baby girl born deaf can hear unaided for the first time, after receiving ground-breaking gene therapy when she was eleven months old at Addenbrooke’s Hospital in Cambridge.
Opal Sandy from Oxfordshire is the first patient treated in a global gene therapy trial, which shows “mind-blowing” results. She is the first British patient in the world and the youngest child to receive this type of treatment.
Opal was born completely deaf because of a rare genetic condition, auditory neuropathy, caused by the disruption of nerve impulses travelling from the inner ear to the brain.
Within four weeks of having the gene therapy infusion to her right ear, Opal responded to sound, even with the cochlear implant in her left ear switched off.
Clinicians noticed continuous improvement in Opal’s hearing in the weeks afterwards. At 24 weeks, they confirmed Opal had close to normal hearing levels for soft sounds, such as whispering, in her treated ear.
Now 18 months old, Opal can respond to her parents’ voices and can communicate words such as “Dada” and “bye-bye.”
Opal’s mother, Jo Sandy, said: “When Opal could first hear us clapping unaided it was mind-blowing – we were so happy when the clinical team confirmed at 24 weeks that her hearing was also picking up softer sounds and speech. The phrase ‘near normal’ hearing was used and everyone was so excited such amazing results had been achieved.”
Auditory neuropathy can be due to a variation in a single gene, known as the OTOF gene. The gene produces a protein called otoferlin, needed to allow the inner hair cells in the ear to communicate with the hearing nerve. Approximately 20,000 people across the UK, Germany, France, Spain, Italy and UK and are deaf due to a mutation in the OTOF gene.
The CHORD trial, which started in May 2023, aims to show whether gene therapy can provide hearing for children born with auditory neuropathy. The trial in Cambridge is being supported by the NIHR Cambridge Clinical Research Facility and the NIHR Cambridge Biomedical Research Centre.

Professor Manohar Bance, NIHR Cambridge BRC researcher and ear surgeon at Cambridge University Hospitals NHS Foundation Trust who is chief investigator of the trial, pictured left, said: “These results are spectacular and better than I expected. Gene therapy has been the future of otology and audiology for many years and I’m so excited that it is now finally here. This is hopefully the start of a new era for gene therapies for the inner ear and many types of hearing loss.”
Children with a variation in the OTOF gene often pass the newborn screening, as the hair cells are working, but they are not talking to the nerve. It means this hearing loss is not commonly detected until children are 2 or 3 years of age – when a delay in speech is likely to be noticed.
Professor Bance added: “We have a short time frame to intervene because of the rapid pace of brain development at this age. Delays in the diagnosis can also cause confusion for families as the many reasons for delayed speech and late intervention can impact a children’s development.”
“More than sixty years after the cochlear implant was first invented – the standard of care treatment for patients with OTOF related hearing loss – this trial shows gene therapy could provide a future alternative. It marks a new era in the treatment for deafness. It also supports the development of other gene therapies that may prove to make a difference in other genetic related hearing conditions, many of which are more common than auditory neuropathy.”
Mutations in the OTOF gene can be identified by standard NHS genetic testing. Opal was identified as being at risk as her older sister has the condition; this was confirmed by genetic test result when she was 3 weeks old.
Opal was given an infusion containing a harmless virus (AAV1). It delivers a working copy of the OTOF gene and is delivered via an injection in the cochlea during surgery under general anaesthesia. During surgery, while Opal was given the gene therapy in right ear, a cochlear implant was fitted in her left ear.
James Sandy, Opal’s father said: “It was our ultimate goal for Opal to hear all the speech sounds. It’s already making a difference to our day-to-day lives, like at bath-time or swimming, when Opal can’t wear her cochlear implant. We feel so proud to have contributed to such pivotal findings, which will hopefully help other children like Opal and their families in the future.”
Opal’s 24-week results, alongside other scientific data from the CHORD trial are being presented at the American Society of Gene and Cell Therapy (ASGC) in Baltimore, USA this week.
Dr Richard Brown, Consultant Paediatrician at CUH, who is an Investigator on the CHORD trial, said: “The development of genomic medicine and alternative treatments is vital for patients worldwide, and increasingly offers hope to children with previously incurable disorders. It is likely that in the long run such treatments require less follow up so may prove to be an attractive option, including within the developing world. Follow up appointments have shown effective results so far with no adverse reactions and it is exciting to see the results to date.
Within the new planned Cambridge Children’s Hospital, we look forward to having a genomic centre of excellence which will support patients from across the region to access the testing they need, and the best treatment, at the right time.”
Martin McLean, Senior Policy Advisor at the National Deaf Children’s Society, said: “Many families will welcome these developments, and we look forward to learning about the long-term outcomes for the children treated. This trial will teach us more about the effectiveness of gene therapy in those cases where deafness has a specific genetic cause.
“We would like to emphasise that, with the right support from the start, deafness should never be a barrier to happiness or fulfilment. As a charity, we support families to make informed choices about medical technologies, so that they can give their deaf child the best possible start in life.”
The CHORD trial is sponsored by Regeneron. Patients are being enrolled in the study in the US, UK and Spain.
Patients in the first phase of the study receive a low dose to one ear. The second phase are expected to use a higher dose of gene therapy in one ear only, following proven safety of the starting dose. The third phase will look at gene therapy in both ears with the dose selected after ensuring the safety and effectiveness in parts 1 and 2. Follow up appointments will continue for five years for enrolled patients, which will show how patients adapt to understand speech in the longer term.
Study highlights increased risk of second cancers among breast cancer survivors
Socioeconomic deprivation linked to greater risk of second cancers among breast cancer survivors
Survivors of breast cancer are at significantly higher risk of developing second cancers, including endometrial and ovarian cancer for women and prostate cancer for men, according to new research studying data from almost 600,000 NHS England patients.
For the first time, the research has shown that this risk is higher in people living in areas of greater socioeconomic deprivation.
Breast cancer is the most commonly diagnosed cancer in the UK. Around 56,000 people in the UK are diagnosed each year, the vast majority (over 99%) of whom are women. Improvements in earlier diagnosis and in treatments mean that five year survival rates have been increasing over time, reaching 87% by 2017 in England.
People who survive breast cancer are at risk of second primary cancer, but until now the exact risk has been unclear. Previously published research suggested that women and men who survive breast cancer are at a 24 and 27% greater risk of a non-breast second primary cancer than the wider population respectively. There have been also suggestions that second primary cancer risks differ by the age at breast cancer diagnosis.
To provide more accurate estimates, a team led by researchers at the University of Cambridge analysed data from over 580,000 female and over 3,500 male breast cancer survivors diagnosed between 1995 and 2019 using the National Cancer Registration Dataset. The results of their analysis are published today in Lancet Regional Health – Europe.
First author Isaac Allen from the Department of Public Health and Primary Care at the University of Cambridge said: “It’s important for us to understand to what extent having one type of cancer puts you at risk of a second cancer at a different site. The female and male breast cancer survivors whose data we studied were at increased risk of a number of second cancers. Knowing this can help inform conversations with their consultants to look out for signs of potential new cancers.”
The researchers found significantly increased risks of cancer in the contralateral (that is, opposite) breast and non-breast second primary cancers for both genders compared to the wider population. The greatest increases in risks were for second cancer of the contralateral breast, and for endometrium and prostate cancer in females and males, respectively.
Females who survived breast cancer were at double the risk of contralateral breast cancer compared to the general population and at 87% greater risk of endometrial cancer, 58% greater risk of myeloid leukaemia and 25% greater risk of ovarian cancer.
Age of diagnosis was important, too – females diagnosed with breast cancer under the age of 50 were 86% more likely to develop a second primary cancer compared to the general population of the same age, whereas women diagnosed after age 50 were at a 17% increased risk. One potential explanation is that a larger number of younger breast cancer survivors carry genetic mutations that increase risk for multiple cancers. For example, women with germline BRCA1 and BRCA2 mutations are at increased risk of contralateral breast cancer, ovarian and pancreatic cancer.
Females from the most socioeconomically deprived backgrounds were at 46% greater risk of a second primary cancer compared to the general population, whereas those from the least deprived backgrounds were at 16% greater risk. These differences were primarily driven by non-breast cancer risks, particularly for lung, kidney, head and neck, bladder, oesophageal and stomach cancers. This may be because smoking, obesity, and alcohol consumption – established risk factors for these cancers – are more common among more deprived groups.
Allen, a PhD student at Clare Hall College, added: “This is further evidence of the health inequalities that people from more deprived backgrounds experience. We need to fully understand why they are at greater risk of second cancers so that we can intervene and reduce this risk.”
Male breast cancer survivors were 55 times more likely than the general male population to develop contralateral breast cancer – though cases were still very rare. They were 58% more likely to develop prostate cancer.
Professor Antonis Antoniou from the Department of Public Health and Primary Care at the University of Cambridge, the study’s senior author, said: “This is the largest study to date to look at the risk in breast cancer survivors of developing a second cancer. We were able to carry this out and calculate more accurate estimates because of the outstanding data sets available to researchers through the NHS.”
The research was funded by Cancer Research UK with support from the National Institute for Health and Care Research Cambridge Biomedical Research Centre.
Reference
Allen, I, et al. Risks of second primary cancers among 584,965 female and male breast cancer survivors in England: a 25-year retrospective cohort study. Lancet Regional Health – Europe; 25 April 2024: DOI: 10.1016/j.lanepe.2024.100903
