Risks from smoking while pregnant more than double previous estimates
Cambridge researchers have shown that women who smoke during pregnancy are 2.6 times more likely to give birth prematurely compared to non-smokers – more than double the previous estimate.
The study analysed data collected during the Pregnancy Outcome Prediction (POP) study, which was supported by NIHR Cambridge BRC, and is published today in the International Journal of Epidemiology.
It also found that smoking meant that the baby was four times more likely to be small for its gestational age, putting it at risk of potentially serious complications including breathing difficulties and infections.
But the team found no evidence that caffeine intake was linked to adverse outcomes.
Women are currently recommended to stop smoking and limit their caffeine intake during pregnancy because of the risk of complications to the baby. For example, smoking during pregnancy is associated with an increased risk of fetal growth restriction, premature birth and low birthweight, though it has also been linked to a reduced risk of preeclampsia (high blood pressure during pregnancy).
High caffeine intake has also been shown to be associated with lower birthweights and possibly fetal growth restriction. Caffeine is more difficult to avoid than cigarette smoke as is found in coffee, tea, chocolate, energy drinks, soft drinks, and certain medications.
Studies looking at the links between smoking, caffeine and adverse pregnancy outcomes tend to rely on self-reported data to estimate exposure, which is not always reliable. A more objective measure is to look at levels of metabolites in the blood – chemical by-products created when substances such as tobacco and caffeine are processed in the body.
Researchers at the University of Cambridge and the Rosie Hospital, part of Cambridge University Hospitals NHS Foundation Trust, recruited more than 4,200 women who attended the hospital between 2008 and 2012 as part of the POP study. The team analysed blood samples taken from a subset of these women four times during their pregnancies.
To assess exposure to cigarette smoke, the team looked at levels of the metabolite cotinine, which can be detected in blood, urine, and saliva. Only two out of three women with detectable levels of cotinine in every blood sample were self-reported smokers, showing that this measure is a more objective way of assessing smoking behaviour.
A total of 914 women were included in the smoking analysis. Of these, 78.6% were classified as having no exposure to smoking while pregnant, 11.7% as having some exposure and 9.7% as having consistent exposure.
Compared to women who were not exposed to smoking while pregnant, those with consistent exposure were 2.6 times more likely to experience spontaneous preterm birth – more than double the previous estimate of 1.27 from a meta-analysis of studies – and 4.1 times as likely to experience fetal growth restriction.
Babies born to smokers were found to be on average 387g lighter than babies born to non-smokers – that is, more than 10% smaller than the weight of an average newborn. This increases the risk that the baby will have a low birth weight (2.5kg or less), which in turn is linked to an increased risk of developmental problems as well as poorer health in later life.
Unlike in previous studies, however, the team found no evidence that smoking reduced the risk of pre-eclampsia.
Professor Gordon Smith, Head of the Department of Obstetrics and Gynaecology at the University of Cambridge, said: “We’ve known for a long time that smoking during pregnancy is not good for the baby, but our study shows that it’s potentially much worse than previously thought. It puts the baby at risk of potentially serious complications from growing too slowly in the womb or from being born too soon.
“We hope this knowledge will help encourage pregnant mums and women planning pregnancy to access smoking-cessation services. Pregnancy is a key time when women quit and if they can remain tobacco free after the birth there are lifelong benefits for them and their child.”
Smoking cessation is offered routinely to all pregnant women and the NHS has local smoking cessation services for anyone, pregnant or not. Further information is available on the NHS website.
To assess caffeine intake, they researchers looked for the metabolite paraxanthine, which accounts for 80% of caffeine metabolism and is both less sensitive to recent intake and more stable throughout the day.
915 women were included in the caffeine analysis. Of these women, 12.8% had low levels of paraxanthine throughout pregnancy (suggesting low caffeine intake), 74.0% had moderate levels and 13.2% had high levels. There was little evidence of an association between caffeine intake and any of the adverse outcomes.
Professor Charlotte Coles receives top honour from Royal College of Radiologists
Congratulations to NIHR Cambridge BRC cancer researcher Professor Charlotte Coles, CRUK RadNet Cambridge lead, on being awarded the Gold Medal by the Royal College of Radiologists.
The Gold Medal is the highest honour that the College can give to a Fellow (radiologist or clinical oncologist) for important work that benefits patients.
Charlotte, who is Professor of Breast Cancer Clinical Oncology and NIHR Research Professor at the University of Cambridge, and Honorary Consultant in Clinical Oncology at Addenbrooke’s Hospital, leads practice-changing research on the best way to deliver radiotherapy treatment to breast cancer patients.
Her research aims to provide breast cancer patients with the best chance of cure with least side effects by personalising radiation techniques based on risk of recurrence.
Charlotte’s work has influenced international hypofractionation policy and she is Chair of the Lancet Breast Cancer Commission, an international multidisciplinary team aiming to influence global policy and improve the lives of people at risk of, and living with, breast cancer.
She leads CRUK RadNet Cambridge, one of seven centres of excellence across the UK pioneering new radiotherapy technologies and techniques to provide better radiotherapy treatments for patients with fewer side effects.
On receiving the Gold Medal at a ceremony at Central Hall Westminster last week, she said: “I feel very honoured and privileged to receive this award on behalf of collaborative patient-centred research in breast cancer and radiation therapy research.”
BRC-supported invention to be showcased at major industry fair
A digital health invention supported by NIHR Cambridge BRC is to be showcased at a major industry fair in London.
BloodCounts!, developed by NHS Blood and Transplant’s Dr Nicholas Gleadall and Dr Michael Roberts at the University of Cambridge, will be presented by NIHR Cambridge BRC partner Cambridge Enterprise at the IP4U University Tech Fair on 19-20 September.
It’s the first time that the Technology Transfer Offices (TTOs) of the University of Cambridge, Imperial College London, Oxford University and University College London have held such an event, which will showcase 80 inventions in sustainability and health.
IP4U will be an opportunity for industry to some of the researchers behind the innovations, and find out how they can partner with academics to commercialise their research.
Early warning system
BloodCounts! uses data from routine blood tests and powerful AI-based techniques to scan for abnormal changes in the blood cells of large populations. Based on this information, doctors can then alert public health agencies to potential emerging infectious disease outbreaks,
The development of the algorithms used in BloodCounts! was only possible due to the EpiCov data-sharing initiative pioneered by Cambridge University Hospitals (CUH) and funded by NIHR Cambridge BRC. The EpiCov database contains de-identified patient and NHS staff data from the CUH Electronic Health Record systems, including scan images and laboratory results.
It includes routinely collected information about patients diagnosed with COVID-19 or suspected of having COVID-19, and staff who have been tested for COVID-19. It also includes information about a large number of control patients who do not have a diagnosis of COVID-19.
- For more information including how to register visit https://ip4u.tech.
Prestigious laureate award for Theme Lead Prof Farooqi
Our Nutrition, Obesity, Metabolism and Endocrinology Theme Lead Professor Sadaf Farooqi has been given the prestigious 2024 Outstanding Clinical Investigator award from the Endocrine Society.
The award honours an internationally recognized clinical investigator who has contributed significantly to understanding the pathogenesis and therapy of endocrine and metabolic diseases. It is one of only a handful of Laureate Awards made by the society each year, to celebrate the achievements of the world’s top endocrinologists.
Professor Farooqi researches the fundamental mechanisms that control human energy homeostasis. She discovered the first genes whose disruption causes severe obesity and established that the principal driver of obesity is a failure of the central control of appetite. She also is a keen advocate to raise more awareness around weight stigma and obesity as a disease.
I am delighted and honoured to receive this prestigious award which recognises the dedication and contributions of past and present team members. I would particularly like to thank the many patients and volunteers who have contributed to our clinical research over the years, allowing us to find new ways to diagnose and treat people with severe obesity.
Professor Farooqi
The Endocrine Society is a global community of physicians and scientists, dedicated to accelerating scientific breakthroughs and improving patient health and well-being. Their main annual meeting, now called ENDO, has been held each year since 1916, except for 1943 and 1945 during World War II. Professor Farooqi will be presented with her award at ENDO 2024 in June next year.
Largest genetic study of brain structure identifies how the brain is organised
The largest ever study of the genetics of the brain – encompassing some 36,000 brain scans – has identified more than 4,000 genetic variants linked to brain structure. The results of the NIHR Cambridge BRC-supported study, led by researchers at the University of Cambridge, are published in Nature Genetics today.
Our brains are very complex organs, with huge variety between individuals in terms of the overall volume of the brain, how it is folded and how thick these folds are. Little is known about how our genetic make-up shapes the development of the brain.
To answer this question, a team led by researchers at the Autism Research Centre, University of Cambridge, accessed MRI scans from over 32,000 adults from the UK Biobank cohort and over 4,000 children from the US-based ABCD study. From these scans, the researchers measured multiple properties of the outermost layer of the brain called the cortex. These included measures of the area and volume of the cortex as well as how the cortex is folded.
They then linked these properties, measured both across the entire cortex as well as in 180 individual regions of the cortex, to genetic information across the genome. The team identified over 4,000 genetic variants linked to brain structure.
These findings have allowed researchers to confirm and, in some cases, identify, how different properties of the brain are genetically linked to each other.
Dr Varun Warrier from the Autism Research Centre, who co-led the study, said: “One question that has interested us for a while is if the same genes that are linked to how big the cortex is – measured as both volume and area – are also linked to how the cortex is folded. By measuring these different properties of the brain and linking them to genetics, we found that different sets of genes contribute to folding and size of the cortex.”
The team also checked whether the same genes that are linked to variation in brain size in the general population overlap with genes linked to clinical conditions where head sizes are much larger or smaller than the general population, known as cephalic conditions.
Dr Richard Bethlehem, also from the Autism Research Centre and a co-lead of the study, said: “Many of the genes linked with differences in the brain sizes in the general population overlapped with genes implicated in cephalic conditions. However, we still do not know how exactly these genes lead to changes in brain size.”
Dr Warrier added: “This work shows that how our brain develops is partly genetic. Our findings can be used to understand how changes in the shape and size of the brain can lead to neurological and psychiatric conditions, potentially leading to better treatment and support for those who need it.”
This study was supported by the Wellcome Trust. It was conducted in association with the NIHR CLAHRC for Cambridgeshire and Peterborough NHS Foundation Trust, and the NIHR Cambridge Biomedical Research Centre.
- Read the paper by Dr Varun Warrier et al, “Genetic insights into human cortical organisation and development through genome-wide analyses of 2,347 neuroimaging phenotypes” in Nature Genetics.
Brain’s ‘appetite control centre’ different in people who are overweight or living with obesity
Cambridge scientists, in research supported by the NIHR Cambridge Biomedical Research Centre, have shown that the hypothalamus, a key region of the brain involved in controlling appetite is different in the brains of people who are overweight and people with obesity when compared to people who are a healthy weight.
The researchers say their findings add further evidence to the relevance of brain structure to weight and food consumption.
Current estimations suggest that over 1.9 billion people worldwide are either overweight or obese. In the UK, according to the Office for Health Improvement & Disparities, almost two-thirds of adults are overweight or living with obesity. This increases an individual’s risk of developing a number of health problems, including type 2 diabetes, heart disease and stroke, cancer and poorer mental health.
A large number of factors influence how much we eat and the types of food we eat, including our genetics, hormone regulation, and the environment in which we live. What happens in our brains to tell us that we are hungry or full is not entirely clear, though studies have shown that the hypothalamus, a small region of the brain about the size of an almond, plays an important role.
Dr Stephanie Brown from the Department of Psychiatry and Lucy Cavendish College, University of Cambridge, said: “Although we know the hypothalamus is important for determining how much we eat, we actually have very little direct information about this brain region in living humans. That’s because it is very small and hard to make out on traditional MRI brain scans.”
The majority of evidence for the role of the hypothalamus in appetite regulation comes from animal studies. These show that there are complex interacting pathways within the hypothalamus, with different cell populations acting together to tell us when we are hungry or full.
To get around this, Dr Brown and colleagues used an algorithm developed using machine learning to analyse MRI brain scans taken from 1,351 young adults across a range of BMI scores, looking for differences in the hypothalamus when comparing individuals who are underweight, healthy weight, overweight and living with obesity.
In a study published today in Neuroimage: Clinical, the team found that the overall volume of the hypothalamus was significantly larger in the overweight and obese groups of young adults. In fact, the team found a significant relationship between volume of the hypothalamus and body-mass index (BMI).
These volume differences were most apparent in those sub-regions of the hypothalamus that control appetite through the release of hormones to balance hunger and fullness.
While the precise significance of the finding is unclear – including whether the structural changes are a cause or a consequence of the changes in body weight – one possibility is that the change relates to inflammation. Previous animal studies have shown that a high fat diet can cause inflammation of the hypothalamus, which in turn prompts insulin resistance and obesity. In mice, just three days of a fat-rich diet is enough to cause this inflammation. Other studies have shown that this inflammation can raise the threshold at which animals are full – in other words, they have to eat more food than usual to feel full.
Dr Brown, the study’s first author, added: “If what we see in mice is the case in people, then eating a high-fat diet could trigger inflammation of our appetite control centre. Over time, this would change our ability to tell when we’ve eaten enough and to how our body processes blood sugar, leading us to put on weight.”
Inflammation may explain why the hypothalamus is larger in these individuals, the team say. One suggestion is that the body reacts to inflammation by increasing the size of the brain’s specialist immune cells, known as glia.
Professor Paul Fletcher, the study’s senior author, from the Department of Psychiatry and Clare College, Cambridge, said: “The last two decades have given us important insights about appetite control and how it may be altered in obesity. Metabolic researchers at Cambridge have played a leading role in this.
“Our hope is that by taking this new approach to analysing brain scans in large datasets, we can further extend this work into humans, ultimately relating these subtle structural brain findings to changes in appetite and eating and generating a more comprehensive understanding of obesity.”
The team say more research is needed to confirm whether increased volume in the hypothalamus is a result of being overweight or whether people with larger hypothalami are predisposed to eat more in the first place. It is also possible that these two factors interact with each other causing a feedback loop.
The research was supported by the NIHR Cambridge Biomedical Research Centre, the Bernard Wolfe Health Neuroscience Fund and Wellcome, with additional funding from Alzheimer’s Research UK.
- View the paper online at Elsevier Science Direct.
Leading professor of paediatric medicine from Canada to visit as part of our Distinguished Lecturer programme
The NIHR Cambridge BRC is delighted to announce that Professor Catherine Birken, Professor of Paediatric Medicine at the University of Toronto, will visit as part of our Distinguished Lecturer Programme this Thursday 21 September.
Professor Birken will deliver a lecture on obesity prevention trials in early childhood, and how interventions from before conception to infancy and early childhood aim to reduce childhood obesity and optimise early child development, nutrition, and other healthy behaviours.
As well as her research in the promotion of healthy growth, development and well-being in young
children, Professor Birken’s clinical care activities include attending children – including those with complex obesity – at the Hospital for Sick Children (SickKids), affiliated with the University of Toronto. This is Canada’s most research-intensive hospital and the largest centre dedicated to improving children’s health in the country.
The Distinguished Lecturer programme is run by the NIHR Cambridge BRC and features world-leading experts and is open to researchers, clinicians and staff working on the Cambridge Biomedical Campus. There are usually two to three lectures a year.
Professor Birken’s lecture will take place from 4-6.45pm on 21 September at the Jeffrey Cheah Biomedical Centre lecture theatre on the campus, ending with a drinks reception.
Registration is not necessary but helpful for catering purposes. If you would like to attend please email lauren.basham@nhs.net by Monday 11 September.
NIHR employees invited to create LGBTQ+ staff network
As part of Pride Month at NIHR, plans have been announced by the NIHR Research Inclusion team to create an NIHR LGBTQ+ Staff Network that will also be open to all NIHR Infrastructure as well as NIHR coordinating centres.
The acronym LGBTQ+ stands for lesbian, gay, bisexual, trans and queer, with the plus encompassing a number of other identities relating to sexual orientation and gender identity including, but not limited to, asexual, pansexual, non-binary, intersex and genderfluid.
NIHR is starting its inclusive staff network journey with an LGBTQ+ Staff Network in response to the interest we received from colleagues during our 2022 Pride activities. The LGBTQ+ Network will serve as a pilot forerunner to other potential NIHR-wide staff networks focusing on different aspects of diversity and inclusion.
Inclusion is one of the NIHR’s operating principles, and embedding inclusion into our systems, processes and culture is one of the areas of strategic focus highlighted in Best Research for Best Health: The Next Chapter.
LGBTQ+ Network membership
The Network will be open to all staff working as part of NIHR (such as staff in coordinating centres, LCRN core teams, delivery staff in LCRN partner organisations and staff in NIHR infrastructure e.g., ARCs and BRCs) who identify as part of the LGBTQ+ community, as well as any staff who consider themselves friends, family or allies to LGBTQ+ people.
Some staff may already be part of LGBTQ+ groups or networks at their employing organisations – the focus of the NIHR LGBTQ+ Staff Network would be to support members around their roles and functions, rather than in relation to their employer.
What is the purpose of the LGBTQ+ Staff Network?
The NIHR Research Inclusion Team is keen to ensure that those within the Network play an active role in deciding what the Network is for.
The NIHR LGBTQ+ Staff Network may provide a space for peer support, developing mentoring relationships and networking across the NIHR for LGBTQ+ staff. The Network may organise events and activities around inclusion of LGBTQ+ people in the work of the NIHR, such as for key dates such as Pride Month, LGBTQ+ History Month, World AIDS Day, Trans Day of Visibility, etc. It may also contribute to activities that support NIHR’s wider strategic inclusion priorities, to ensure inclusivity around sexuality and gender identity in all aspects of the work of the NIHR.
How do we find out more or get involved?
You can get involved in one of two ways:
- register your interest in joining a small working group to help us determine the aims and focus of the LGBTQ+ Staff Network and how it will work
- register your interest in joining the Network once it has been designed.
If you are interested in being part of the Network in either or both of the above capacities, please complete this short form by 14 July 2023 for the NIHR Research Inclusion Team.
Contact:
Please contact the NIHR Research Inclusion Team if you have any further questions or comments.
- Visit the About Us section on our website to find out more about Nurturing Inclusive Research at NIHR Cambridge BRC.
Researchers awarded prestigious Academy of Medical Sciences Fellowships
Four NIHR Cambridge BRC researchers have been elected to the Academy of Medical Sciences Fellowship.
Theme Leads Professors James Rowe and Serena Nik-Zainal, together with researchers Professors Charlotte Coles and Emanuele Di Angelantonio, received the awards in recognition of their outstanding biomedical and health research which has translated into benefits for patients and wider society.




Academy of Medical Sciences President Professor Dame Anne Johnson said: “These new Fellows are pioneering biomedical research and driving life-saving improvements in healthcare. It’s a pleasure to recognise and celebrate their exceptional talent by welcoming them to the Fellowship.”
- This year Fellows were chosen from 353 candidates, and a shortlist of 126 candidates for peer review. To find out more about the Fellowship visit the Academy of Medical Sciences website.
Cambridge researcher wins prestigious award for rare eye disease gene replacement therapy research
Neuroscience researcher Professor Patrick Yu-Wai-Man has won the 2023 Ludwig von Sallmann Clinician-Scientist Award from the Association for Research in Vision and Ophthalmology (ARVO) Foundation.
The award was given in recognition of Prof Yu-Wai-Man’s research on gene replacement therapy for Leber hereditary optic neuropathy (LHON).
LHON is a genetic disorder caused by mutations in mitochondrial DNA (mtDNA). Strictly inherited down the maternal line, it is an important cause of inherited blindness in the young adult population. Currently, there are limited treatment options for LHON and most affected individuals will remain within the legal criteria for blindness.
Prof Yu-Wai-Man said: “I have been working in the field of mitochondrial diseases for nearly 25 years and despite the amazing advances made during that period, finding effective treatments has proven challenging.
“Mitochondrial optic neuropathies have led the way and this award is a recognition of the translational breakthroughs seen in recent years, in particular gene replacement therapy for Leber hereditary optic neuropathy.”
Prof Yu-Wai-Man has worked on several studies using a modified version of the MT-ND4 mitochondrial gene packaged into an adeno-associated viral vector (AAV2) that is injected into the eye. Promising results have been obtained for individuals treated within one year of disease onset with a significant and sustained improvement in vision observed during long-term follow-up.
On receiving the Ludwig von Sallmann Clinician-Scientist Award, Prof Yu-Wai-Man said: “It is a great honour and my hope is that this award will highlight the significant unmet needs for individuals affected with mitochondrial optic neuropathies, which result in significant visual impairment in children and young adults.
“We need to attract more research funding and talents into rare genetic eye diseases. Success breeds success and the future certainly looks bright in this field.”
This article is adapted from arvo.org
Three more great events at this year’s Cambridge Festival – and they’re all from BRC researchers
We’ve already publicised our webinar and family event taking place as part of this year’s Cambridge Festival – now we want to let you know about three more events taking place during the Festival, and they’re all from researchers we fund and support.
Check out the details below – and don’t forget to find out about our events (if you haven’t already) and other NIHR events taking place in Cambridge.
Mental Health
The crisis in mental health in young women and girls: does our education system make it worse? What should we do?
Why are girls and young women suffering levels of stress and anxiety so far in excess of those their mothers and grandmothers experienced? What part does stress at school and university play? Do we have too many exams? Is the content of university courses too onerous? Does the method of assessment at UK universities stress out young women? Why are university students more likely to suffer anxiety than their counterparts at work?
Join Professors Sarah-Jayne Blakemore and Tamsin Ford as they examine some potential solutions, in this hour-long talk from 6-7pm on Monday 27 March, taking place at the Babbage Lecture Theatre, Downing Street.
To find out more and book your place.
Imaging
New medical imaging techniques in the era of A.I.
Dr Joshua Kaggie is an MRI physicist and senior research associate in the Department of Radiology, where he works on developing new imaging techniques. Dr Kaggie moved to Cambridge from Utah in 2015 and has been involved in a range of projects including osteoarthritis, cancer, and dementia imaging.
This talk will discuss some of the more novel imaging techniques that are being developed at Addenbrooke’s Hospital, including the use of heavy hydrogen (deuterium) for use as a new cancer imaging method. This talk will discuss artificial intelligence / machine learning (AI/ML) techniques, their current and future impact on medical imaging and diagnostics, and medical imaging research underway at Addenbrooke’s Hospital – with an emphasis on MRI techniques. The talk will feature a live demonstration of interesting AI developments, some of which may not relate to medicine – yet.
The talk is on Monday, 27 March, from 7.30-8.15pm, at University of Cambridge Admissions Office, New Museums site, Bene’t Street. For more information and to book your place.
Neurodegenerative Disease and Dementias
Memory Matters – an in-person discussion about brain health
This event, taking place from 3.30-5.00pm on Thursday 30 March in the Herchel Smith Building, Robinson Way, will be an opportunity to join researchers for a discussion about brain health in the context of ageing and dementia.
Many of us are worried about our memory as we get older, or following stress to the body (such as long COVID), and it is common to wonder whether we might be developing dementia. But, how much do you know about dementia and how it is detected and treated? In this workshop, you will have the chance to meet, and ask questions of, the doctors, psychologists and nurses who run the world-famous Addenbrooke’s Hospital Memory Clinic.
Short presentations will be followed by a live Q&A session with members of the Memory Clinic team.
For more information and details on how to book.

NICE recommends Cambridge-developed ‘artificial pancreas’ for use on NHS for management of type 1 diabetes
The artificial pancreas for type 1 diabetes – which was developed in Cambridge and supported in trials by the NIHR Cambridge BRC and NIHR Cambridge CRF – could soon be approved for use in the NHS.
The technology is now being recommended by the National Institute for Health and Care Excellence (NICE) as a new way of controlling diabetes and if approved, could be a life-changing tool to manage the disease.
Currently, people with type 1 diabetes rely on multiple, daily finger-prick blood tests and insulin injections to manage their blood sugar, because their pancreas no longer produces insulin.
This new technology combines an insulin pump, continuous glucose monitor and an algorithm to calculate and deliver the right amount of insulin needed. NICE has recommended that the device is offered to patients whose diabetes is difficult to control with other technologies and who are at increased risk of long-term complications – around 105,000 people in England and Wales.
Trialling a new technology
The NIHR Cambridge BRC and CRF have supported the artificial pancreas research from the earliest phases of research more than ten years ago. The device was trialled at the CRF in multiple stages with hundreds of patients, including pregnant women and children.
Research nurses collected blood samples and glucose readings and even conducted overnight studies to understand how effective the device would be to patients whilst they slept. Studies found the artificial pancreas was beneficial in managing people’s diabetes.
Professor Roman Hovorka, who led the team that developed the artificial pancreas, said: “NICE’s recommendations are very welcome and it comes after years of randomised controlled trials (RCTs) at Cambridge.
“These provided the necessary clinical and economic evidence that showed the device has clear health benefits and potential cost savings.
“This technology can literally change lives. If blood glucose levels are too low or too high it can be very damaging and even life-threatening.
“Our trials showed that using the device improved patients’ quality of life and reduced the risk of long-term health complications.”
The closed-loop algorithm developed in Cambridge is now available through CamDiab in 15 countries worldwide, including Australia, France, Italy and Poland.
- See also Artificial pancreas successfully trialled for use by type 2 diabetes patients.
- Artificial pancreas proves ‘life-changing’ for very young children with type 1 diabetes and their families
Gone fishing: highly accurate test for common respiratory viruses uses DNA as ‘bait’
Research supported by NIHR Cambridge BRC has led to a new test that ‘fishes’ for multiple respiratory viruses at once using single strands of DNA as ‘bait’, giving highly accurate results in under an hour.
Cambridge researchers developed the test, which uses DNA ‘nanobait’ to detect the most common respiratory viruses – including influenza, rhinovirus, RSV and COVID-19 – at the same time.
In contrast, PCR (polymerase chain reaction) tests, while highly specific and highly accurate, can only test for a single virus at a time and take several hours to return a result.
While many common respiratory viruses have similar symptoms, they require different treatments. By testing for multiple viruses at once, the researchers say their test will ensure patients get the right treatment quickly and could also reduce the unwarranted use of antibiotics.
In addition, the tests can be used in any setting, and can be easily modified to detect different bacteria and viruses, including potential new variants of SARS-CoV-2, the virus which causes COVID-19. The results are reported in the journal Nature Nanotechnology.
The winter cold, flu and RSV season has arrived in the northern hemisphere, and healthcare workers must make quick decisions about treatment when patients show up in their hospital or clinic.
Similar symptoms, different treatments
“Many respiratory viruses have similar symptoms but require different treatments: we wanted to see if we could search for multiple viruses in parallel,” said Filip Bošković from Cambridge’s Cavendish Laboratory, the paper’s first author. “According to the World Health Organization, respiratory viruses are the cause of death for 20% of children who die under the age of five. If you could come up with a test that could detect multiple viruses quickly and accurately, it could make a huge difference.”
For Bošković, the research is also personal: as a young child, he was in hospital for almost a month with a high fever. Doctors could not figure out the cause of his illness until a PCR machine became available.
“Good diagnostics are the key to good treatments,” said Bošković, who is a PhD student at St John’s College, Cambridge. “People show up at hospital in need of treatment and they might be carrying multiple different viruses, but unless you can discriminate between different viruses, there is a risk patients could receive incorrect treatment.”
PCR tests are powerful, sensitive and accurate, but they require a piece of genome to be copied millions of times, which takes several hours.
The Cambridge researchers wanted to develop a test that uses RNA to detect viruses directly, without the need to copy the genome, but with high enough sensitivity to be useful in a healthcare setting.
“For patients, we know that rapid diagnosis improves their outcome, so being able to detect the infectious agent quickly could save their life,” said co-author Professor Stephen Baker, from the Cambridge Institute of Therapeutic Immunology and Infectious Disease. “For healthcare workers, such a test could be used anywhere, in the UK or in any low- or middle-income setting, which helps ensure patients get the correct treatment quickly and reduce the use of unwarranted antibiotics.”
The researchers based their test on structures built from double strands of DNA with overhanging single strands. These single strands are the ‘bait’: they are programmed to ‘fish’ for specific regions in the RNA of target viruses. The nanobaits are then passed through very tiny holes called nanopores. Nanopore sensing is like a ticker tape reader that transforms molecular structures into digital information in milliseconds. The structure of each nanobait reveals the target virus or its variant.
The researchers showed that the test can easily be reprogrammed to discriminate between viral variants, including variants of the virus that causes COVID-19. The approach enables near 100% specificity due to the precision of the programmable nanobait structures.
“This work elegantly uses new technology to solve multiple current limitations in one go,” said Baker. “One of the things we struggle with most is the rapid and accurate identification of the organisms causing the infection. This technology is a potential game changer; a rapid, low-cost diagnostic platform that is simple and can be used anywhere on any sample.”
A patent on the technology has been filed by Cambridge Enterprise, the University’s commercialisation arm, and co-author Professor Ulrich Keyser has co-founded a company, Cambridge Nucleomics, focused on RNA detection with single-molecule precision.
“Nanobait is based on DNA nanotechnology and will allow for many more exciting applications in the future,” said Keyser, who is based at the Cavendish Laboratory. “For commercial applications and roll-out to the public we will have to convert our nanopore platform into a hand-held device.”
“Bringing together researchers from medicine, physics, engineering and chemistry helped us come up with a truly meaningful solution to a difficult problem,” said Bošković, who received a 2022 PhD award from Cambridge Society for Applied Research for this work.
- The research was supported in part by the European Research Council, the Winton Programme for the Physics of Sustainability, St John’s College, UK Research and Innovation (UKRI), Wellcome and the NIHR Cambridge Biomedical Research Centre.
Cambridge researchers launch study to investigate the impact of neonatal intensive care on premature babies’ sleep patterns and brain development
Cambridge researchers funded by Action Medical Research and the NIHR Cambridge BRC are investigating the impact interruptions in sleep cycles – such as loud noises – have on the development of brain activity in preterm babies in neonatal intensive care (NICU).
The study is significant as preterm babies develop in an environment that is very different from the womb.
The frequent and often painful procedures, bright lights and loud noises in NICU interrupt the natural sleep cycles which are essential for normal brain development – and there is increasing evidence that the different sleep cycles in babies, known as active and quiet sleep, play an important role in the early development of the brain.
Preterm babies to wear ‘swimming caps’ containing sensors to measure brain activity during sleep
In this study, changes in blood flow and oxygen levels will be measured in different parts of the brain using a non-invasive wearable technology, similar to a swimming cap, worn on the head during the quiet and active sleep cycles of very preterm and healthy term infants. This optical imaging technology is called high-density diffuse optical tomography (HD-DOT), and it images functional connections in the brain at the cot-side.
Consultant Neonatologist Professor Topun Austin, who is also Director of the NIHR Cambridge BRC facility the Evelyn Perinatal Imaging Centre, said: “The results from this study could have long-term implications for the care of preterm babies, as this will increase understanding of the sleep states that promote the development of the brain.
“Ultimately this could lead to a traffic light system next to cots, with a red light to indicate a baby is in active or quiet sleep and should not be disturbed, and a green light when the baby is transitioning between sleep states, indicating that the baby can be woken up for tests, feeding or other care.”
In previous research in healthy term infants, Professor Austin and his team showed there was a significant difference in functional connectivity in different sleep states – with babies in active sleep having more connections crossing the brain hemispheres compared with quiet sleep.
This research is needed as in the UK one in every 13 babies are born too soon – before 37 weeks of pregnancy – and around 8,000 babies are born before 32 weeks. Advances in treatment have led to improved survival, however preterm babies have an increased risk of long-term neurodevelopmental complications. Very preterm babies, born before 32 weeks, are at a higher risk of developing behavioural and emotional problems and poor sleep.
- World Prematurity Day takes place each year on 17 November.
- Professor Austin has been interviewed about this research by MIT Technology Review – read the article here.
Off-patent liver disease drug could prevent COVID-19 infection
Cambridge scientists have identified an off-patent drug that can be repurposed to prevent COVID-19 – and may be capable of protecting against future variants of the virus – in research part-funded by the NIHR Cambridge BRC.
The research, published in Nature, showed that an existing drug used to treat a type of liver disease is able to ‘lock’ the doorway by which SARS-CoV-2 enters our cells, a receptor on the cell surface known as ACE2. Because this drug targets the host cells and not the virus, it should protect against future new variants of the virus as well as other coronaviruses that might emerge.
If confirmed in larger clinical trials, this could provide a vital drug for protecting those individuals for whom vaccines are ineffective or inaccessible as well as individuals at increased risk of infection.
Dr Fotios Sampaziotis, from the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge and Addenbrooke’s Hospital, led the research in collaboration with Professor Ludovic Vallier from the Berlin Institute of Health at Charité.
Dr Sampaziotis said: “Vaccines protect us by boosting our immune system so that it can recognise the virus and clear it, or at least weaken it. But vaccines don’t work for everyone – for example patients with a weak immune system – and not everyone have access to them. Also, the virus can mutate to new vaccine-resistant variants.
“We’re interested in finding alternative ways to protect us from SARS-CoV-2 infection that are not dependent on the immune system and could complement vaccination. We’ve discovered a way to close the door to the virus, preventing it from getting into our cells in the first place and protecting us from infection.”
Work on organoids
Dr Sampaziotis had previously been working with organoids – ‘mini-bile ducts’ – to study diseases of the bile ducts. Organoids are clusters of cells that can grow and proliferate in culture, taking on a 3D structure that has the same functions as the part of the organ being studied.
Using these, the researchers found that a molecule known as FXR, which is present in large amounts in these bile duct organoids, directly regulates the viral ‘doorway’ ACE2, effectively opening and closing it. They went on to show that ursodeoxycholic acid (UDCA), an off-patent drug used to treat a form of liver disease known as primary biliary cholangitis, ‘turns down’ FXR and closes the ACE2 doorway.
In this new study, his team showed that they could use the same approach to close the ACE2 doorway in ‘mini-lungs’ and ‘mini-guts’ – representing the two main targets of SARS-CoV-2 – and prevent viral infection.
The next step was to show that the drug could prevent infection not only in lab-grown cells but also in living organisms. For this, they teamed by up with Professor Andrew Owen from the University of Liverpool to show that the drug prevented infection in hamsters exposed to the virus, which are used as the ‘gold-standard’ model for pre-clinical testing of drugs against SARS-CoV-2. Importantly, the hamsters treated with UDCA were protected from the -new at the time- delta variant of the virus, which was new at the time and , which was -at least partially- resistant to existing vaccines.
Professor Owen said: “Although we will need properly-controlled randomised trials to confirm these findings, the data provide compelling evidence that UDCA could work as a drug to protect against COVID-19 and complement vaccination programmes, particularly in vulnerable population groups. As it targets the ACE2 receptor directly, we hope it may be more resilient to changes resulting from the evolution of the SARS-CoV-2 spike, which result in the rapid emergence of new variants.”
Testing using donated lungs not suitable for transplantation
Next, the researchers worked with Professor Andrew Fisher from Newcastle University and Professor Chris Watson from Addenbrooke’s hospital to see if their findings in hamsters held true in human lungs exposed to the virus.
The team took a pair of donated lungs not suitable for transplantation, keeping them breathing outside the body with a ventilator and using a pump to circulate blood-like fluid through them to keep the organs functioning while they could be studied. One lung was given the drug, but both were exposed to SARS-CoV-2. Sure enough, the lung that received the drug did not become infected, while the other lung did.
Professor Fisher said: “This is one of the first studies to test the effect of a drug in a whole human organ while it’s being perfused. This could prove important for organ transplantation – given the risks of passing on COVID-19 through transplanted organs, it could open up the possibility of treating organs with drugs to clear the virus before transplantation.”
Testing on healthy volunteers
Moving next to human volunteers, the Cambridge team collaborated with Professor Ansgar Lohse from the University Medical Centre Hamburg-Eppendorf in Germany.
Professor Lohse explained: “We recruited eight healthy volunteers to receive the drug. When we swabbed the noses of these volunteers, we found lower levels of ACE2, suggesting that the virus would have fewer opportunities to break into and infect their nasal cells – the main gateway for the virus.”.
While it wasn’t possible to run a full-scale clinical trial, the researchers did the next best thing: looking at data on COVID-19 outcomes from two independent cohorts of patients, comparing those individuals who were already taking UDCA for their liver conditions against patients not receiving the drug. They found that patients receiving UDCA were less likely to develop severe COVID-19 and be hospitalised.
A safe, affordable variant-proof drug
First author and PhD candidate Teresa Brevini from the University of Cambridge said: “This unique study gave us the opportunity to do really translational science, using a laboratory finding to directly address a clinical need.
“Using almost every approach at our fingertips we showed that an existing drug shuts the door on the virus and can protect us from COVID-19. Importantly, because this drug works on our cells, it is not affected by mutations in the virus and should be effective even as new variants emerge.”
Dr Sampaziotis said the drug could be an affordable and effective way of protecting those for whom the COVID-19 vaccine is ineffective or inaccessible. “We have used UDCA in clinic for many years, so we know it’s safe and very well tolerated, which makes administering it to individuals with high COVID-19 risk straightforward.
“This tablet costs little, can be produced in large quantities fast and easily stored or shipped, which makes it easy to rapidly deploy during outbreaks – especially against vaccine-resistant variants, when it might be the only line of protection while waiting for new vaccines to be developed. We are optimistic that this drug could become an important weapon in our fight against COVID-19.”
The research was largely funded by UK Research & Innovation, the European Association for the Study of the Liver, the NIHR Cambridge Biomedical Research Centre and the Evelyn Trust.
- Watch a video of Dr Sampaziotis explaining the implications of this research:
Cambridge researchers develop safe, affordable device for prostate cancer diagnosis
A new medical device developed at Addenbrooke’s and supported by the NIHR Cambridge BRC and NIHR Cambridge Clinical Research Facility aims to reduce the risk of infection in prostate patients – and save time and money.
The device – called the Cambridge Prostate Biopsy Device (CamPROBE) – has been developed by urology specialist Professor Vincent Gnanapragasam and his team at Cambridge University Hospitals and Cambridge University.
Traditionally, prostate patients have had transrectal biopsies, where a sample of tissue is removed from the prostate using a thin needle that is inserted through the rectum and into the prostate.
This carries a significant risk of side effects, including urinary infections and severe sepsis -and medical and professional bodies now advocate using instead the transperineal route, which is the space between the legs and under the scrotum.
The CamPROBE uses the transperinal route – making it safer for patients. It’s also cost-effective and simple to use – the procedure can be carried out in outpatients under local anaesthetic.
Urology consultant Professor Gnanapragasam (pictured below) said: “In trials cancer detection rates were equivalent to other means of biopsy.
“Procedure times were short and only low amounts of local anaesthetic were required, yet low pain scores were reported by patients.

“More than 85% of patients said they would recommend the CamPROBE procedure as a method of having a prostate biopsy done.”
The CamPROBE aims to make the lives of patients better through a simple and low pain approach of prostate cancer detection, hopefully benefitting the millions of men who have prostate biopsies every year.
A licensing agreement for CamPROBE has been agreed with product development company JEB Technologies.
NIHR statement on the death of Her Majesty Queen Elizabeth II
We at the NIHR offer our sincerest condolences to the royal family on the passing of Her Majesty Queen Elizabeth II. We share in the grief of the whole nation at the death of our remarkable monarch, and reflect with great pride upon her incredible reign.
Research reveals how genetic mutations cause kidney cancer
Researchers at the University of Cambridge have shown that genetic mutations associated with kidney cancer rely on factors that regulate normal kidney cells in order to develop into cancer cells.
The study suggests that similar mechanisms could explain why cancer mutations cause specific types of cancer to develop.
Inherited genetic variants and mutations that are randomly acquired during the lifetime of an individual can lead to the development of cancer. But different mutations tend to cause different types of cancer.
While this cancer-specificity of mutations has been clear for decades, exactly why mutations can lead to the formation of tumours in some tissues but not others has remained poorly understood.
All tissues have specific functions that are ultimately dependent on the instructions encoded by the genome. These instructions are read by a group of proteins called transcription factors that recognise specific DNA sequences and ensure that the right regions of the genome are active in each cell. This mechanism allows the same genome to control the functions of diverse cell types with vastly different characteristics.
In this study, published in the journal Nature, the researchers tested whether the transcription factors that control tissue-specific functions of normal kidney cells were also required for the growth of kidney cancers.
They used a combination of advanced genomic tools, experimental cancer models and analysis of large human data sets.
The results show that the ability of kidney cancer-associated genetic alterations to promote tumour formation was dependent on transcription factors that are specifically active in normal kidney cells.
When kidney-specific transcription factors were inactivated experimentally, the cancer mutations were no longer capable of activating genes that are important for tumour growth.
Senior author Dr Sakari Vanharanta said: “Our results provide some insight into the molecular mechanisms that dictate the cancer type-specificity of mutations.
“Genetic alterations that cause kidney cancer rely on factors that under normal conditions regulate specific functions of healthy kidney cells. If these factors are not present, as they are not in most other cell types, the process that eventually leads to cancer formation does not proceed.”
Large genetic alterations commonly observed in advanced, metastatic kidney cancer cells also relied on kidney-specific transcription factors for their cancer-promoting effect.
The common genetic variant characterised in this study is carried by the majority of individuals of European descent and it increases the risk of kidney cancer. Overall, the molecular mechanisms described in this work are likely to be important for a large proportion of kidney cancers.
Professor Grant Stewart, study co-author and co-lead of the CRUK Cambridge Centre Urological Malignancies Programme, said: “[This] paves the way to new thinking on how to develop new treatments for kidney cancer and even prevent it from developing in the first place.
“These same mechanisms might also be at play in other cancer types, making this study highly relevant across all cancers”.
Cambridge researchers to receive nearly £4m to tackle cancer roadblocks
NIHR Cambridge BRC researchers are among the Cambridge scientists to receive £3,938,500 as part of Cancer Grand Challenges, a major initiative co-founded by Cancer Research UK and the National Cancer Institute in the US, which aims to encourage the world’s leading cancer researchers to take on some of the toughest challenges in cancer research.
The eDyNAmiC (extrachromosomal DNA in Cancer) team will investigate new ways to combat treatment resistant cancers, while the CANCAN (CANcer Cachexia Action Network) team hopes to prevent cachexia, where patients ‘waste away’ in the later stages of their disease.
Funding for the Cambridge-based projects is part of an overall £80 million awarded this week to four elite global teams who will deepen our understanding of cancer through international collaboration leading to new advances for people with cancer.
Extrachromosomal DNA – present in up to a third of all tumours, helps to evade treatment
The Stanford University-led eDyNAmiC team, which includes Professor Serena Nik Zainal at the University of Cambridge, hopes to tackle tumour evolution, which is driven by circular pieces of tumour DNA which exist outside the tumour and pose a major problem by enabling tumours to resist treatment.
Research is now revealing that a major driver of tumour evolution is extrachromosomal DNA (ecDNA). These small circular DNA particles enable cells to rapidly change their genomes and so evade the immune system.
EcDNA doesn’t follow the rules of normal chromosomes, providing tumours a way to evolve and change their genomes to evade treatment.
Although first observed in cancer in 1965, researchers are only beginning to understand the extent to which it is prevalent in around a third of cancers and how it helps tumours to become more resistant, aggressive and affect patient survival.
The goal of eDyNAmiC is to understand how extrachromosomal DNA is created, to find vulnerabilities and then to develop new ways to target these in some of the hardest cancers to treat, including glioblastoma, lung and oesophageal cancer.
Co-investigator Professor Nik-Zainal (pictured, below), of the University of Cambridge Early Cancer Institute and Department of Medical Genetics, said: “My team and I are so excited to be part of this collaboration studying this phenomenon of extra pieces of DNA called ecDNA.
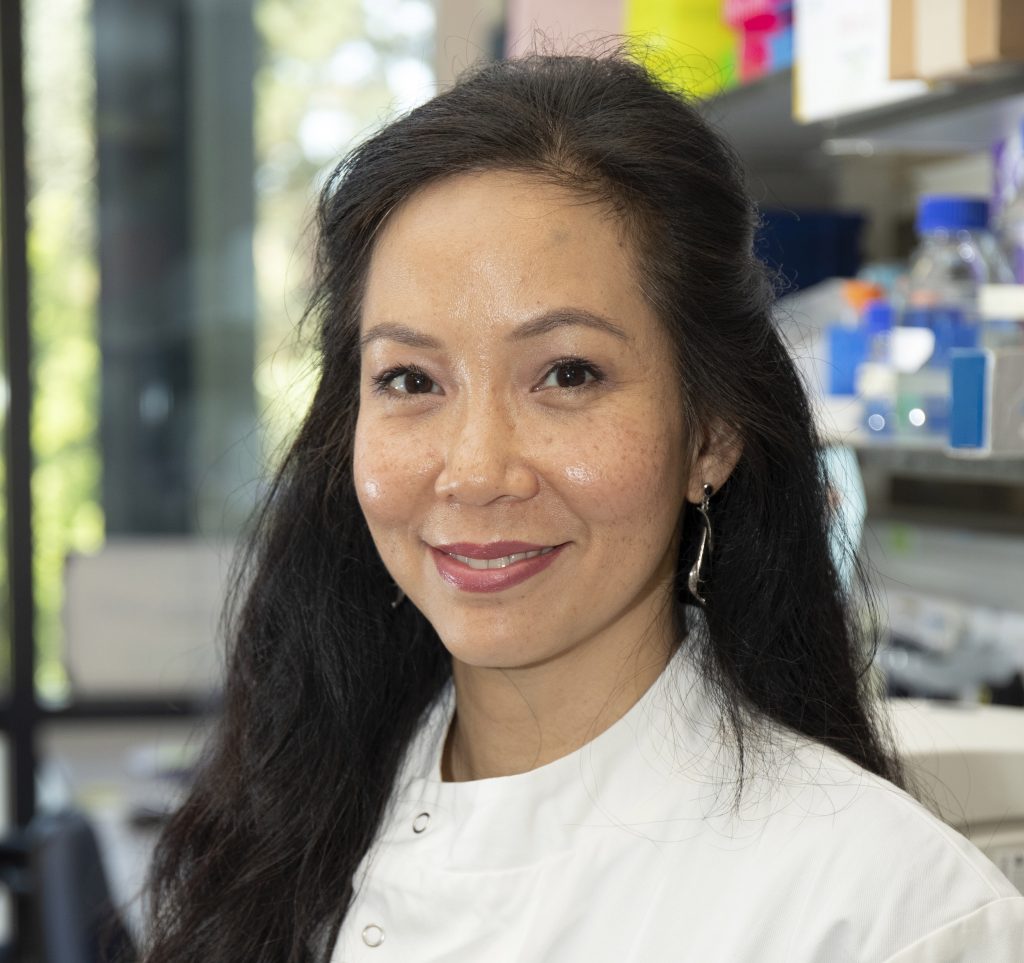
“What a privilege it is to be entrusted to explore how ecDNAs cause cancer and drive them to be aggressive. We hope that what we learn will bring real benefits to cancer patients in due course.”
Research aims to improve quality of life for patients with cancer cachexia
The CANCAN team is led by US researchers who will work with co-investigators Professor Sir Stephen O’Rahilly and Dr Tony Coll, of the Wellcome-MRC Institute of Metabolic Science, and Dr Giulia Biffi, of the CRUK Cambridge Institute, to explore the underpinning mechanisms behind cancer cachexia – a debilitating wasting condition many people experience in the later stages of the disease.

Cachexia syndrome is characterised by poor appetite and extensive weight loss from both skeletal muscle and fatty tissue and is still not fully understood.
It is hoped further research can help develop new treatments to improve quality of life for cancer patients and set the standard for cachexia management around the world.
Professor Sir Stephen O’Rahilly said: “For many decades we have studied how a range of hormones act on the brain to regulate appetite and body weight.
“Many of the insights that we have gained through our previous research in obesity are likely to be highly relevant to cancer cachexia, a condition where hormonal and metabolic changes secondary to cancer impact on the brain to reduce, rather than increase appetite.
“We have recently discovered a pathway in the brain which is key to controlling whether we put food calories in excess of our basic needs into fat or into muscle. This pathway is likely to be highly relevant to patients with cancer cachexia who are particularly affected by a loss of muscle.”
Dr Giulia Biffi’s research focuses on understanding the biology of the tumour microenvironment in pancreatic cancer with the aim of developing new treatments and diagnostics.
Dr Biffi, who co-leads the CRUK Cambridge Centre Pancreatic Cancer Programme, said: “Cachexia is predominant in pancreatic cancer patients; it increases patient mortality and can prevent patients accessing treatment as they are too weak. If we can identify ways to treat cachexia more people could be treated for their cancer.
“This is a fantastic opportunity to interact with a highly multi-disciplinary team to bring our different scientific and clinical strategies towards a single common goal.”
- Dr Coll with Dr Claire Connell have recently opened a clinical study at Addenbrooke’s hospital which aims to understand the mechanisms underlying weight loss in cancer patients by investigating changes to metabolism and the immune system. They hope the findings from the Metabolic and Immunological Phenotyping in Patients with Cancer (MIPPaC) study will guide future research and help to design treatments that can alleviate or prevent weight loss and improve outcomes for cancer patients.
Consensus opens door to worldwide improvements in breast cancer radiotherapy treatment
A panel of European experts and patients has identified a way to achieve major changes in the way radiotherapy treatment for breast cancer patients is delivered around the world.
The Consensus from ESTRO–ACROP: European Society for Radiotherapy and Oncology – Advisory Committee in Radiation Oncology Practice, which has been published online by Lancet Oncology, states that 3-week breast radiotherapy can be offered to any patient for any indication and 1-week breast radiotherapy can be offered for patients who do not need lymph node radiotherapy.
It follows months of work from the panel. They first looked at research on increased doses of radiation over a shorter timeframe (called hypofractionation), and then compiled consensus statements.
These were reviewed by experts from hospitals across Europe, Brazil and Israel, who graded how much they either agreed or disagreed with the statements.
Finally, the panel selected the statements that scored the highest and agreed a formal Consensus.
Professor Charlotte Coles, who is also an NIHR Research Professor, Deputy Head of the Department of Oncology at the University of Cambridge and a member of the core ESTRO-ACROP consensus group, said: “In the clinical world Consensus statements can drive change – so this really is the next step to changing standard of care in Europe and the rest of the world.
“We knew the evidence was there to support hypofractionation – there have been some excellent clinical trials over the last couple of decades. These include the recent UK FAST-Forward trial that showed that 1-week breast radiotherapy is at least as good as conventional treatment in preventing cancer returning with similar side effects, but has far fewer treatment visits for patients.”
Hypofractionation is already standard of care in the UK, but it has been much slower to implement elsewhere. In addition, there are huge areas of the world, for example in parts of Africa, where access to radiotherapy treatment is extremely poor.
Professor Coles said: “Changing from three or even five weeks in some places to one week will make breast radiotherapy treatment a realistic goal for everyone who needs it.
“There are other benefits too, including less travel for treatment, less time off work, less time in recovery.
“What the Consensus is saying is that doctors could treat three breast cancer patients in the time that it used to take to treat just one, which would mean more patients could access breast radiotherapy.
“The good things about reducing the number of weeks is we’re not giving less treatment, it’s the same treatment only over one week, in more concentrated doses.
“The science behind it is based on how breast cancer reacts to hypofractionation, and it’s a happy consequence that it’s also quicker and cheaper.”
Professor Coles is delighted that the Consensus will be published in Lancet Oncology: “This is the first time that a consensus or guideline from ESTRO-ACROP has been published in a high-impact journal like Lancet Oncology.
“This will help us enormously in our work to influence policy makers to ensure equity of access to evidence-based radiotherapy.
“We know Consensus statements drive change but in many places health care providers are still reimbursed for each dose they deliver and not for the whole course. This is a disincentive for providing evidence-based hypofractionation and needs to be changed.
“We want equity so that everyone can access the radiotherapy they need and for providers to be incentivised by the quality of the radiotherapy they deliver rather than the number of treatments so that the patient experience is better.
“I am currently chairing The Lancet Breast Cancer Commission, which will look at reimbursement as part of its remit. We hope this Consensus will convince policy-makers of the health and economic value of breast hypofractionation.
“The ultimate goal is to make high-quality breast cancer radiotherapy accessible for everyone, no matter where they live.
“We’ve already changed practice in the UK, we now need to change it internationally.”
- “European Society for Radiotherapy and consensus recommendations on patient selection and dose and fractionation for external beam radiation therapy in early breast cancer” will appear in print in Lancet Oncology in January 2022.
