Facilities

The NIHR Cambridge BRC has embedded essential infrastructure on the Cambridge Biomedical Campus making sure we translate our world-leading science into real benefits for healthcare.
Click on the facilities below to find out more about what they can offer to help your research:
- Brain Bank
- Cambridge Clinical Research Centre (CCRC) including NIHR Cambridge Clinical Research Facility
- Cambridge Clinical Trials Unit
- Cambridge Clinical Vision Laboratory
- Cambridge Enterprise
- Clinical Trials Pharmacy
- Core Biochemical Assay Laboratory (CBAL)
- The Evelyn Perinatal Imaging Centre
- Gut Reaction
- NIHR BioResource for Translational Research in Common and Rare Diseases
- NIHR Cambridge BRC Cell Phenotyping Hub
- Office for Translational Research
- PET/CT Merck
- SMCL Next Generation Sequencing (NGS) Hub

Brain Bank
The Cambridge Brain Bank was established to enable brain tissue to be used after death for research into neurodegenerative disorders such as Dementia (Alzheimer’s, Frontotemportal etc.), Motor Neurone disease, Huntington’s disease, Multiple Sclerosis, Parkinson’s disease etc.
The donation of post-mortem brain tissue for research is of fundamental importance to further understanding of the causes of these disorders and to develop more effective diagnostic tools and treatments for these conditions.

Cambridge Clinical Research Centre (CCRC)
The CCRC provides state-of-the-art, clinical research facilities to support world class research in a wide range of clinical conditions in patients of all ages, translating cutting edge science into the medicines and treatments of the future.
The NIHR Cambridge Clinical Research Facility is a 24/7 inpatient unit with a Metabolic Research Area containing measurement technologies for energy balance and body composition allied to metabolic magnetic resonance spectroscopy in the Wolfson Brain Imaging Centre. This CRF has dedicated facilities to accommodate children.
The Clinical Investigation Ward (CIW) operates on a day case basis, undertaking later-phase clinical trials.
With a five floor expansion of campus clinical research capacity, facilities include:
- Level 2: Interventional Investigation Unit: Facilities to undertake research endoscopies; cell therapy and minor procedures
- Level 3: Early Phase Trials Unit: Dedicated facilities for early phase studies including first-in-human trials
- Levels 4 and 6: Wellcome-MRC Metabolic Translational Research Facility: Conducts metabolic studies linked to research programmes funded by the Wellcome Trust-MRC Institute of Metabolic Science (IMS). With dedicated space and equipment for studying patients and volunteers in a naturalistic setting
- Level 5: Clinical Research Facility – a 24/7 unit accommodating research in diverse specialties in adult patients and volunteers.
To contact the CCRC call: 01223 254800.

Cambridge Clinical Trials Unit
The Cambridge Clinical Trials Unit (CCTU) was established in 2011 and is hosted at the Cambridge University Hospitals NHS Foundation Trust as part of the Cambridge Biomedical Research Centre.
The CCTU works with researchers from Cambridge University Health Partners (CUHP) to conduct the highest quality clinical research, addressing important questions related to human health and disease. Major areas within our portfolio include: oncology, cardiovascular disease, infection and immunity, paediatrics, neurology, neurosciences and trauma, imaging, surgery and peri-operative care.
We strive to deliver world class clinical trials ranging from national to international, single to multi-centre, CTIMPS, non-CTIMPs and medical device trials. The CCTU supports all trial stages, from study design, costing grant proposals, developing protocols, obtaining necessary approvals, delivery and quality control, to data management, final analysis and dissemination.
The CCTU is part of the NIHR UKCRC Registered CTU Network, and receives NIHR CTU Support Funding to develop and conduct NIHR trials. The CCTU is also a member of the NCRI Cancer CTU. We are a founding member of the International Clinical Trials Centre Network, which aims to improve clinical research by fostering collaborations worldwide.
- Find out more details of the CCTU’s studies.
- Call the CCTU on 01223 596474.

Cambridge Clinical Vision Laboratory (CCVL)
The Cambridge Clinical Vision Laboratory (CCVL) provides access to the latest technology for advanced ocular phenotyping. Our vision is to drive forward innovation and support clinical trials on the Cambridge Biomedical Campus, with a particular focus on gene therapy and cell-based therapies for ocular and neurodegenerative diseases.
The CCVL is a dedicated facility for vision research embedded within the NIHR Cambridge Clinical Research Facility in a strategic location next to the Early Phase Trials Unit.
The CCVL is part of a major drive by the NIHR Cambridge BRC to fast-track advanced gene therapy and cell-based therapies for ocular and neurodegenerative diseases. We also support early-phase experimental trials exploring novel chemotherapeutic and immunomodulatory agents for cancer and various autoimmune diseases.
The CCVL is available to assist and support your studies, including grant applications. Please contact us to discuss your ideas and requirements further.

Cambridge Enterprise
Part of the University of Cambridge, Cambridge Enterprise supports its members in achieving knowledge transfer and research impact.
We help transform your ideas into commercial opportunities attractive to organisations that can bring them to market and support formation of new companies and social enterprises.
We also manage the commercial relationship and negotiation of relevant agreements. Our remit extends to supporting the development of intellectual property arising from NIHR Cambridge BRC activities and collaborations between the University of Cambridge and the NHS; if you are either a CUH NHS Trust employee, or a University employee supported by the NIHR Cambridge BRC, and want to discuss how to commercialise your technology, we have the experience and expertise to support you.
If you are ready to disclose your idea confidentially, then please complete our Idea Disclosure form.
If you would like to ask us a question, please email CBRCIPSupport@enterprise.cam.ac.uk, and someone from the team will get in touch with you.

Clinical Trials Pharmacy
Pharmacy support for clinical trials is provided by a dedicated team of pharmacists, technicians and support officers who work together with Trust colleagues and external sponsors to support a range of both commercial and non-commercial studies involving Investigational Medicinal Products (IMPs).
The team also have growing experience with Advanced Therapy IMPs and work closely with Cambridge Cellular Therapy Laboratory to provide pharmaceutical governance for studies involving cellular treatments.
Pharmacy clinical trial support is an integral part of the research process and has a vital role to play in safeguarding participants, healthcare professionals and the Trust by ensuring IMPs and other study medications are appropriate for use and are procured, handled, stored and used safely and correctly in line with regulatory requirements. A dedicated Clinical Trials Dispensary is on site and pharmacy aseptic dispensing facilities are also available.
For Trust-sponsored Clinical Trials of IMPs (CTIMPs), pharmacy are involved from the outset of a project and work closely with Cambridge Clinical Trials Unit to advise on costings for grant applications, protocol design and review of the overall trial application in relation to medication, including manufacturing and participating site aspects. Pharmacy will also advise on non-CTIMP research as required.
- Call the Clinical Trials Pharmacy on 01223 596233.

Core Biochemical Assay Laboratory (CBAL)
CBAL offers a high quality cost-effective analytical service. Since its inception in 2008 the laboratory has worked closely with many research groups across the Cambridge Biomedical campus and helped commercial companies develop and evaluate their novel assays.
The majority of the 300+ different tests offered by CBAL use immunoassay technology. CBAL performs MesoScale Discovery, Luminex Magpix, DELFIA and ELISA assays plus those available on the Siemens Dimension EXL, Diasorin Liaison XL and Randox Daytona+ Autoanalysers.
CBAL has a good track record of developing novel high quality assays for biomarkers that are not readily available from commercial vendors.
The laboratory is located on Level 4 of the Addenbrooke’s Laboratory block. It currently has CPA-accreditation for its two most requested ‘clinical’ assays (insulin & c-peptide); other assays are unaccredited but performed to the highest standard. CBAL is staffed by up to seven highly experienced NHS Biomedical Scientists, funded by the NIHR Cambridge Biomedical Research Centre. All staff have received GCP training and participate in CPD schemes.
- Download the CBAL Users’ Handbook (updated July 2020).
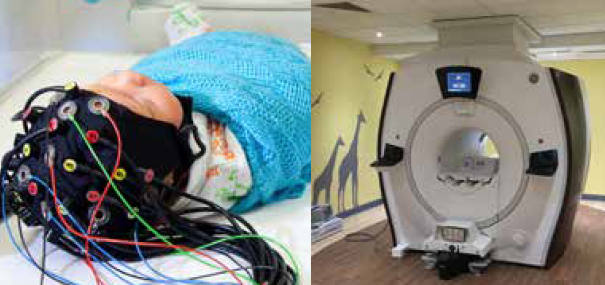
The Evelyn Perinatal Imaging Centre
The Evelyn Perinatal Imaging Centre is a unique clinical research facility, dedicated to studying brain function and development in newborn babies to inform clinical practice.
Its internationally-competitive programme of research brings together clinicians, engineers and physicists to develop new technologies to study the causes, detection and diagnosis of brain injury in newborns.
Located in the Rosie maternity hospital, close to the neonatal intensive care unit (NICU), postnatal wards and birthing suite, the centre houses a physics laboratory, clinical 1.5T MRI scanner and infant scanning room with dedicated MRI-compatible incubator, making it safer and easier to transfer sick and preterm infants.
The centre enables researchers and clinicians to study and care for critically-ill babies in their first hours of life, in an infant-friendly and safe environment. Enhanced clinical care means babies’ outcomes have the best chance of improving and also facilitates new lines of neuroprotective treatments.
Through its ties with the University’s Department of Paediatrics and with NIHR Cambridge BRC support, the Evelyn Perinatal Imaging Centre is playing a key role in developing neonatal neurocritical care (‘Neuro-NICU’), to improve the quality of care for newborn infants with congenital and acquired brain injury. The centre is also closely involved with The Next Generation Children’s project, which is undertaking whole genome sequencing on infants with altered neurology and seizures.
The centre has enabled a number of successful commercial, industrial and academic collaborations in Cambridge, London and Europe, including the development of a 3D optical imaging system and state-of-the-art EEG monitoring systems. Together these systems provide a unique multimodal imaging facility to study brain development in the newborn, identify those at risk of long-term brain injury and better understand how babies respond to different treatments.
To find out how this facility can help your research or to enquire about opportunities for collaboration, please contact Centre Director Professor Topun Austin. For more information on the centre and current research projects, visit https://neolabresearch.com.

Gut Reaction
Gut Reaction is the world’s largest repository of high quality, consented and linkable data supporting research to change the lives of people living with inflammatory bowel disease (IBD).
Gut Reaction was funded for an initial 3-year period by Health Data Research UK (HDR UK), through the UK Research and Innovation (UKRI) Industrial Strategy Challenge Fund (ISCF), to facilitate research into IBD by bringing together datasets from different sources.
From September 2022, Gut Reaction will continue as part of the NIHR Bioresource. Working with their partners, collaborators and the IBD community, they will continue to support research that makes a difference to those living with IBD. You can browse or apply to access available datasets by visiting their data pages and learn more about how they work with the IBD community on their patient pages.
If you would like to get in touch with the Gut Reaction team, please email them on gutreaction@bioresource.nihr.ac.uk

NIHR BioResource for Translational Research in Common and Rare Diseases
Headquartered in Cambridge, the NIHR BioResource is an umbrella organisation with local centres in Birmingham, Cambridge, Exeter, Leeds, Leicester, Manchester, Newcastle and Southampton and London – including Barts, Guy’s and St Thomas’, Maudsley, Moorfields and UCLH. The aim of the NIHR BioResource is to facilitate research by helping researchers with volunteer recruitment and recall specifically by genotype and or phenotype.
The NIHR BioResource has its own Clinical Research Unit at Addenbrooke’s hospital on the Cambridge Biomedical Campus. It is a purpose-built facility to support clinical research for the NIHR BioResource. Facilities include three clinical rooms, a clinical prep room and a family room to enable volunteer participation.
When researchers apply to the NIHR BioResource or our NIHR Cambridge BioResource Centre for recall studies, they can request support from the BioResource Clinical team and or other local BioResource Centres across England. Please see the below pictures of the NIHR Cambridge BRC Clinical Research Unit.
Clinical research nurses support studies requiring a range of clinical tasks including venepuncture, skin biopsies, biometric measurements and other clinical procedures with appropriate training as required.
The BioResource Centre Cambridge is one of the local sites run at the NIHR Cambridge BRC.

NIHR Cambridge BRC Cell Phenotyping Hub
NIHR Cambridge BRC Cell Phenotyping Hub, established in 2011 for rapid processing and analysis of human samples operating under the direction of Anna Petrunkina-Harrison, is a major component of the BRC infrastructure and simultaneously part of the cross-cutting Inflammation, Infection and Immune-therapeutics research theme.
The Hub’s mission is to deliver advanced scientific technologies for supporting the excellence in research of a partnership between the University of Cambridge and Cambridge University Hospitals NHS Foundation Trust.
The Hub provides state-of-the-art resources and cost-effective high quality scientific, educational and technological expertise and services across the wide range of cytomics and immune phenotyping. These services fall mainly into three major areas: research service, clinical services and technology services. They include but are not limited to fluorescence-activated and magnetic cell isolation and purification, single cell analysis, confocal imaging, sample preparation (laser capture microdissection and blood processing) and comprehensive phenotyping from sample to data. The Hub is equipped with state-of-the-art equipment including high-speed cell sorters, bench-top analysers and high content/high throughput equipment.
A further function of the Hub is to collect and to disseminate the unique technological and scientific expertise crucial for promoting and supporting patient-based basic research and for translating basic biological findings into clinical practice. The Hub drives technological innovations and applies technical and instrumental advances to expand the portfolio of services and fulfill the emerging research needs. Our group consists of research and support scientists and technologists with a long history record of research and development and with commensurate levels of scientific and technical expertise. Through our collaborative Research and Development service we foster and fortify collaborations that result in many major publications across nearly all Departments of the Clinical School and BRC themes.
The main Hub laboratories are located in the Jeffrey Cheah Biomedical Centre and on Levels 5 and 6 of the Addenbrooke’s main hospital block. Additional outlet is being established in the laboratories of HLRI. For access to the Hub services, all new clients and prospective collaborators are invited to attend a Hub induction to discuss their research needs and to customise service provision (contact via e-mail below).
- Call 01223 330149
- Back to top

Office for Translational Research (OTR)
The Office for Translational Research (OTR) can help convert research ideas coming from the University of Cambridge and associated hospitals into new products and health applications to treat or prevent human disease and illness.
The OTR provides:
- Partnering and project advice
- Project advice at all stages (“Bench to Bedside”) and big initiatives
- Training and Events
The OTR receives core-funding from the NIHR Cambridge Biomedical Research Centre and the University of Cambridge’s HEFCE Higher Education Innovation funding (HEIF).
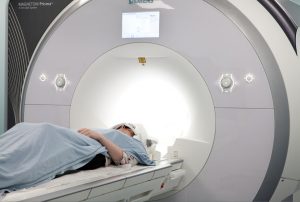
PET/CT Merck
Cambridge is fortunate to have excellent clinical cross-sectional functional imaging facilities with research access to four 1.5T, three 3T Magnetic resonance imaging (MRI) systems, four multi-detector (computed tomography) CTs (16, 64 and 128 multi-detector), dual source and dual energy CT, more than eight colour flow Doppler US systems, several gamma cameras and positron emission tomography (PET)-CT.
The MRC clinical infrastructure funding has provided exciting new opportunities with the upgraded 3T and new 7T MRI, PET-MR and a second clinical hyperpolariser. In addition, an upgraded and extended GMP PET radiopharmaceutical unit with an on-site cyclotron and state-of-the-art radiochemistry systems with the capability of producing a large range of short-lived and longer-lived PET radiotracers.
These installations will particularly facilitate and enhance experimental therapeutic trials in dementia, oncology, cardiovascular medicine and within other themes in the NIHR Cambridge BRC.

SMCL Next Generation Sequencing (NGS) Hub
The Stratified Medicine Core Laboratory (SMCL) offers researchers access to clinical grade next generation sequencing; a range of platforms to suit all budgets and coverage requirements at short turnaround times. SMCL can provide a complete ‘end to end’ service with a fully flexible approach, encompassing nucleic acid extraction and sample QC, full library preparation and sequencing and bioinformatics analysis or support (or individual parts of this pathway).
The Stratified Medicine Core Laboratory (SMCL) NGS Hub is involved in world class sequencing research partnerships with clients both within the University of Cambridge and the Cambridge University Hospital NHS Foundation Trust, along with further external Academic and Commercial organisations.
The SMCL NGS Hub aims to provide a comprehensive and expert Next Generation Sequencing service to the academic, clinical & commercial community, with the distinctive mission of providing a clinical translational service.
The SMCL NGS Hub is hosted by the Department of Medical Genetics of the University of Cambridge and, uniquely, housed in the Medical Genetics Laboratories of the East Midlands and East of England NHS Genomic Laboratory Hub (East GLH), allowing us to offer researchers access to clinical grade next generation sequencing services.
What can SMCL offer to the academic, clinical and commercial communities?
- SMCL offers researchers access to clinical grade next generation sequencing; a range of platforms to suit all budgets and coverage requirements at short turnaround times.
- SMCL can provide a complete ‘end to end’ service with a fully flexible approach, encompassing nucleic acid extraction and sample QC, full library preparation and sequencing and bioinformatics analysis or support (or individual parts of this pathway).
- SMCL also offers advice on experimental and project design, and can tailor services (e.g., bespoke NGS assays) to specific project needs.
- SMCL’s typical services include:
- Extraction services
- Library Prep
- Clinical Grade Whole Genome Sequencing
- Clinical Grade Whole Exome Sequencing
- Clinical Grade Targeted Sequencing
- RNA-SEQ
- Epigenetic analysis (targeted methylation assays, genome wide methylation profiling, RRBS).
- SMCL equipment
- Illumina sequencers
- Illumina cBot
- Agilent Tapestation
- QIAGEN QIAsymphony
- Beckman Coulter Biomek FXP
- Covaris E220 Focused Ultrasonicator
Please visit SMCL website for further details about the facility’s services and equipment.
For quotes and general enquiries, please contact SMCL by email.


